Archive : Article / Volume 1, Issue 1
- Review Article | DOI:
- https://doi.org/10.58489/2836-5917/003
Coronavirus Fulminant Myocarditis May Mimic Acute MI: Case Review
Klinikum Nurnberg Hospital, Germany.
N John Camm
N John Camm, (2022). Coronavirus Fulminant Myocarditis May Mimic Acute MI: Case Review. Clinical Cardiovascular Research. 1(1). DOI: 10.58489/2836-5917/003
© 2022 N John Camm, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 31-08-2022
- Accepted Date: 12-09-2022
- Published Date: 29-11-2022
Retrosternal pain, Elevated cardiac markers and Electrocardiographic ST-T changes.
Abstract
The present study describes the case of a woman aged 48 who had acute retrosternal pain, elevated cardiac markers and electrocardiographic ST-T changes, which led to an original misdiagnosis of acute myocardial infarction. The patient underwent immediate coronary angiography, which revealed normal coronary arteries. Finally, the diagnosis of viral myocarditis was made on consideration of his fever, scattered red dots on his arms and legs and other auxiliary examination results obtained in the following days, which were supportive of the diagnosis. The patient improved on antiviral and myocardial protection therapy and was discharged 2 weeks later. Viral myocarditis is a common disease with a variable natural history. It remains challenging for doctors to differentiate between acute myocarditis and myocardial infarction, particularly in the early stages. A diagnosis of myocarditis should be made on the basis of synthetic evaluation of the evidence, including medical history, clinical presentation and results of the available auxiliary tests, in order to provide guidelines for treatment.
Clinical picture:
A 48-year-old female patient was admitted to the hospital on, 2020, with chest pain and dyspnea for 7 days, accompanied by acute Gasterornteritis. Her blood pressure (BP) dropped to 90/60mm Hg.
Investigation:
Chest X-ray revealed enlargement of the heart (cardiothoracic ratio, 0.70).
Chest CT examination indicated lung infection, cardiomegaly, and pleural effusion.
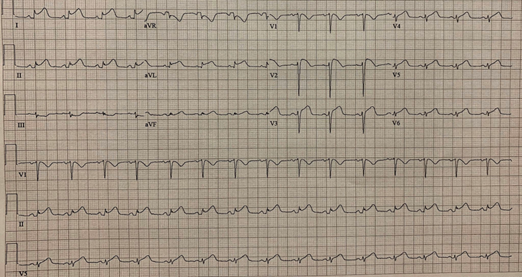
The ECG suspected ST-segment elevation myocardial infarction (III, AVF ST-segment elevation). ECG points to mid LAD occlusion. This topographic ST elevation is not usual in myocarditis.
CT coronary angiography revealed normal coronary.
Markers of myocardial injury: Troponin T was >10.000 ng/L. Creatine kinase isoenzyme (CK-MB) = 12.9 ng/L. B-type natriuretic peptide (BNP) = 2,1025 ng/L.
Echocardiography revealed an enlarged heart and a marked decrease in ventricular systolic function (left ventricular end-diastolic dimension [LVEDD] 58 mm, left atrium (LA) 39 mm, right ventricle (RV) 25 mm, right atrium (RA) 48 mm, LV ejection fraction [LVEF] 27%, trace 2 mm pericardial effusion).
Sputum was examined for 13 viral nucleic acids related to respiratory tract. Only the coronavirus nucleic acid test was positive. All of the other 12 nucleic acid tests were negative, including influenza A virus, adenovirus, bocavirus, rhinovirus, influenza A (H1N1) 2009, parainfluenza, chlamydia, partial pulmonary virus, influenza B virus, mycoplasma pneumoniae, influenza A virus H3N2, and respiratory syncytial virus.
Diagnosis by the authors of this case report: Fulminant coronavirus myocarditis with pulmonary infection.
Therapy included:
- Methylprednisolone. to suppress inflammation (200 mg/day, 4 days), and immunoglobulin to regulate immune status (20 g/day, 4 days)
- Norepinephrine to raise BP
- Diuretic therapy (torsemide and furosemide) to reduce cardiac load
- Milrinone to increase myocardial contractility
- Piperacillin sulbactam for infection
- Pantoprazole, to inhibit gastric acid
Clinical course
After treatment, the patient’s symptoms improved significantly.
One week later:
- X-ray chest film showed heart size normal (cardiothoracic ratio, 0.49).
- Echocardiography showed that the size and function of the heart had returned to normal (LVEDD 42 mm, LA 34 mm, RV 24 mm, RA 33 mm, LVEF 66%).
Markers of myocardial injury:
- After 1 week of treatment: Troponin T was 220.5 ng/L. CK-MB was 9.14 ng/L. BNP was 1587 ng/L.
- After 3 weeks, the myocardial injury markers had fully recovered to the normal range. Troponin T was 21.4 ng/L. CK-MB was 2.25 ng/L. BNP was 139 ng/L.
Differential Diagnosis:
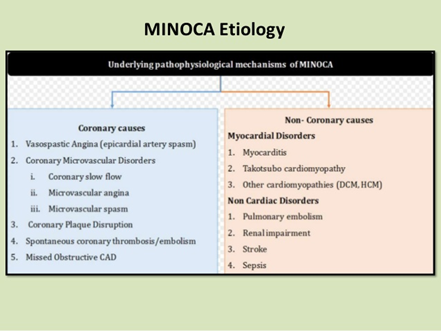
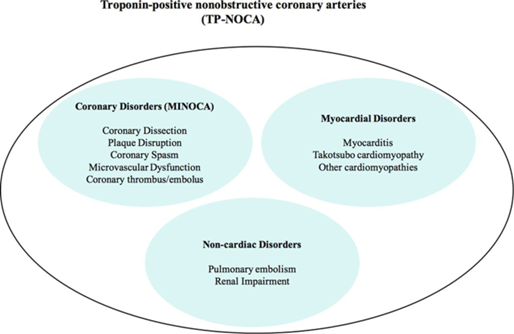
- Myocardial infarction with non-obstructive coronary arteries (MINOCA) is clinically defined by the presence of the universal acute myocardial infarction (AMI) criteria, absence of obstructive coronary artery disease (≥50% stenosis), and no overt cause for the clinical presentation at the time of angiography.
- Takotsubo cardiomyopathy.
Discussion:
Viral myocarditis is a common disease with a variable natural history. In the present case, the patient had typical retrosternal pain, elevated cardiac markers and electrocardiographic ST-T changes on admission, and was initially diagnosed with acute myocardial infarction; however, emergency coronary angiography demonstrated normal
coronary anatomy. In addition, in the week following admission the patient presented with a flu-like illness manifesting as slight chills and fever. His cardiac enzymes subsequently decreased and the ST-segment elevation gradually decreased. Other unique characteristics of myocarditis in this case were the temperature fluctuations and the rash on the patient's arms and legs, which appeared later during his hospitalization. Based on these clinical manifestations and auxiliary examination, the diagnosis of acute myocarditis could be confirmed.
It is likely that the typical clinical presentations of myocardial infarction, such as chest pain, ST-segment elevation and incremental serum markers, appear in patients diagnosed with myocarditis. The diagnosis of myocarditis is often empirical. Physicians should take into consideration several lines of evidence, such as clinical presentation, ECG alterations and cardiac enzyme changes, prior to making a diagnosis. In addition, epicardial coronary artery disease should be excluded. The following discussion aims to provide a comprehensive description of viral myocarditis, including the pathogenesis, clinical presentation, development of diagnostic methods and treatment.
With regard to the etiology of the condition, the advancement of molecular biology has led to the identification of a number of different viruses and virus subtypes that are causative factors for myocarditis. The more common viruses are coxsackievirus, adenovirus, cytomegalovirus and parvovirus B19, as well as hepatitis C, influenza, herpes simplex and Epstein-Barr viruses.
In the first phase of myocarditis, the virus enters and proliferates in the myocardium, causing direct myocardial damage, followed by the initiation of the innate immune response. Both the direct myocardial damage caused by the virus and the subsequent immune process result in destruction of the cardiomyocytes and lead to elevations in the serum cardiac Tn and enzyme levels, mainly to eliminate as many virus-infected cells as possible to control the infection, which includes the activation of complement (a process that produces both cell lysis and substances chemotactic for neutrophils and macrophages), the activation of various cytokines and the infiltration of T lymphocytes and macrophages, which can be detected by biopsy. Following the first phase, patients will recover or progress into the second phase in which the adaptive immune response is activated.
Molecular mimicry accounts for part of the persisting myocardial damage in the second phase, as the virus antigens and the myocardial cells share similar epitopes, which activate the B cells to produce cross-reacting antibodies and thus activate the effector T cells. Lawson et al. induced persisting myocarditis in the susceptible BALB/c strain of mice with mouse cytomegalovirus, and found that autoantibodies to cardiac myosin were produced following mouse cytomegalovirus infection. These affinity-purified anti-cardiac myosin antibodies cross-reacting with mouse cytomegalovirus proteins suggest that viral infection may modulate the immune recognition of the common epitopes shared between the mouse cytomegalovirus proteins and the heavy chain of myosin. Cross-reacting antibodies with auto-antigens have also been found in patients with myocarditis. In the third phase, the intensity of the immune response is down regulated and fibrosis starts. As a result, the persistent low-grade immune response leads to extensive myocardial injury and, eventually, dilated cardiomyopathy.
The clinical manifestations of viral myocarditis are highly variable, ranging from asymptomatic to acute heart failure. Acute myocarditis often presents with a flu-like illness, including fever, myalgia, malaise, nausea and vomiting, for a few days to 3 weeks before any cardiac symptoms appear. The majority of patients will make a full recovery; however, a number of patients can rapidly progress to chest pain, respiratory distress, arrhythmia or even heart failure, which necessitates hospital admission. Further physical examination may reveal cardiac pathological signs, such as sinus tachycardia, low first heart sounds, gallops and murmurs of mitral or tricuspid insufficiency, which are not specific for myocarditis. Other unspecific signs, such as the appearance of skin rashes, can also be found in certain patients. In the current case of viral myocarditis, the patient had fever with temperature fluctuations between 35.5 and 38.4°C and subsequently showed a rash on the arms and legs in the week following admission.
Several diagnosis modalities can be helpful in the diagnosis of myocarditis, including electrocardiography and cardiac biomarkers. Damage of the myocytes causes abnormal electrical activity of the heart, which leads to abnormalities in the ECG, including ST-T wave changes, ST elevation, atrial and ventricular arrhythmias, atrial-ventricular and intraventricular conduction defects and variant early repolarization. however, these ECG alterations are non-specific. Myocarditis may share similar ECG changes with myocardial infarction. TnT, TnI and creatine kinase (CK)-MB are the most commonly used cardiac biomarkers. Cardiac Tn is mainly elevated in the acute phase of myocarditis and decreases gradually as the patient improves. The sensitivity of cardiac biomarkers to myocardial injury varies. As with electrocardiography, cardiac Tns are non-specific for myocarditis, although they are more sensitive than CK levels. Consistently, in this case of viral myocarditis, the TnI level began rising from the first day of admission, peaked at 8.470 ng/ml on the third day but then slumped and reached 0.295 ng/ml on the ninth day. ST-segment elevations in the II, III and aVF leads on the ECG, accompanied by acute retrosternal pain and elevated cardiac markers, led to the initial incorrect diagnosis of myocardial infarction.
Since only certain patients present with elevated cardiac enzymes, the reliability of cardiac enzymes for diagnosing myocarditis remains uncertain and should be investigated further. In addition to cardiac Tns, the level of brain natriuretic peptide (BNP) measured in the plasma may be a useful biochemical marker for myocarditis, and high concentrations of BNP may correlate with poor prognosis in patients with myocarditis. Caforio et al (22) suggested that the log-BNP concentration could be a quantitative biochemical marker of myocarditis in Kawasaki disease. Viral culture should be considered to help identify the virus responsible for the disease, although the virus can only be isolated from the blood in a minority of cases. Polymerase chain reactions, immunoglobulin antibody assays and viral titers can help to improve the possibility of detecting the pathogen.
Echocardiography can also be of use in the diagnosis of myocarditis. The echocardiographic findings suggestive of myocarditis are left ventricular dilation, decreased function, systolic and diastolic dysfunction and regional wall motion abnormalities. Furthermore, patients may have myocardial interstitial edema, which can also be detected by echocardiography through the thickness of the ventricular wall. There are additionally non-specific echocardiographic characteristics associated with acute myocarditis. In the present study, normal heart function was suggested by an echocardiogram. Developments in technology have brought new progress in diagnosis. Notably, recent reports have recommended that speckle-tracking echocardiography, characterized by the precise evaluation of regional contractility, should be used as an adjunctive tool for the diagnosis of acute myocarditis and inflammatory cardiomyopathy.
In recent years, cardiac magnetic resonance imaging (CMRI) has emerged as one of the most useful imaging devices for detecting and diagnosing myocarditis, as it can provide various means to visualize and quantify myocardial inflammatory changes CMRI, however, is not accepted by a proportion of patients with suspected myocarditis due to the considerable expense. The current patient was one such case.
Finally, the histological and immunological evaluation of biopsies can be used in the diagnosis of acute myocarditis. Endomyocardial biopsy (EMB) is not a routine diagnostic method in the majority of cases of suspected acute myocarditis, since it is an invasive approach and has a probability of sampling error due to the characteristic patchy inflammation and variability in observer interpretation. A 2013 position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases recommended heart biopsy as a routine test for all cases of suspected myocarditis. Conversely, the routine application of EMB was not recommended by the 2013 American College of Cardiology Foundation/American Heart Association, therefore, no consensus has been reached with regard to the application of EMB in the diagnosis of myocarditis. EMB was not performed in the present case.
Advances in histological and molecular genetic technology, such as the polymerase chain reaction and in situ hybridization, have improved the efficiency of identifying viral genomes and cardiac inflammation. According to the Canadian Cardiovascular Society Consensus Conference guidelines on heart failure (updated 2009), EMB evaluation for myocarditis should include the use of histopathological markers of inflammation and necrosis, immunohistochemical markers and the assessment of viral particles, however, it has been indicated that the histological diagnosis of myocarditis based on the Dallas criteria lacks sensitivity and specificity, Additionally, an absence of sensitive markers for an active immunological process can limit the use of histopathological analysis, Immunohistochemical techniques can enable the quantification and identification of activated inflammatory cells, including T lymphocytes, B cells, macrophages and natural killer cells. Among those cells, T lymphocytes are essential for diagnosing active myocarditis. Immunostains for cell-specific markers may also help confirm the presence of myocardial inflammation. The analysis of viral replication in the myocardium through the use of the polymerase chain reaction and in situ hybridization can help quantify the specific viral variants accounting for myocardial damage. Previous findings using these novel diagnostic tests point toward a broader spectrum of viral genomes responsible for acute myocarditis, indicating a shift from enterovirus and adenovirus to parvovirus B19 and human herpes-virus 6 as the viruses most frequently causing acute myocarditis.
With regard to the treatment of the condition, ~50% of the patients with acute myocarditis are likely to spontaneously recover within a month, ~25% will develop persistent impaired cardiac function and up to 25–30% may either progress to dilated cardiomyopathy, making heart transplantation a necessity, or succumb to the condition.
For symptomatic treatment of acute myocarditis, physical activity should be avoided, as sports may promote viral replication and increase the burden on the heart. As long as the patient has symptoms such as chest pain, respiratory distress, ECG abnormalities, increased levels of TnI/T or CK-MB, symptomatic treatment should be undertaken, including diuretics, β-blockers, angiotensin-converting enzyme-inhibitors or angiotensin II receptor blockers. Patients presenting with heart failure should be administered suitable drugs, including positive inotropic agents, vasodilators and diuretics. In the case of severe heart failure, mechanical circulatory support should be introduced, such as an intra-aortic balloon pump or a left ventricular assist device.Heart transplantation should be considered if the aforementioned measures fail. Arrhythmia is common, particularly ventricular arrhythmia. If patients present with severe refractory ventricular arrhythmias or atrioventricular blocks, they may require antiarrhythmic medication or the insertion of implantable cardioverter defibrillators or temporary pacemakers, respectively.
Since patients are generally diagnosed with myocarditis within days or weeks after the initial viral infection, antiviral therapy is seldom used in clinical practice in the early phase of myocarditis, although antiviral therapy has been reported to have a positive effect in the acute viremic stage. A patient with parvovirus-B19-associated fulminant myocarditis was reported to show a complete recovery with immunosuppressive and antiviral therapy (intravenous immunoglobulin and acyclovir) within 2 weeks. Furthermore, the antiviral effect of interferon-β therapy, enhanced by the transcription suppressor 4E-BP1, has been reported in myocarditis induced by coxsackievirus B3. Consistently, in the present study, the patient was administered the antiviral drug acyclovir and cardiac metabolism-promoting drugs and recovered within 2 weeks.
Immune suppression may be beneficial in patients with systemic disease-related or autoimmune myocarditis but may increase virus replication and worsen myocardial injury in viral myocarditis. A recent study showed that immunosuppressive treatment taken immediately on detection of sclerotic heart disease proved effective in preventing cardiac damage progression. Similarly, an improved performance of immunosuppressive therapy (azathioprine and prednisone) was reported for children with chronic myocarditis, regardless of the presence of viral infection, compared with conventional therapy. By contrast, Hia et al reviewed the impact of immunosuppressive therapy on the outcome of acute myocarditis in children, and the 18 years of data suggested that immunosuppressive therapy does not significantly improve outcomes in children with acute myocarditis, providing negative evidence for its routine use. It is therefore necessary to evaluate the type of myocarditis carefully prior to starting the immunosuppressive therapy in order to avoid its ill effects.
Despite considerable progress, it remains a daunting challenge for physicians to discriminate between acute myocarditis and myocardial infarction, particularly in the early phase. An integrated assessment and evaluation of evidence, including medical histories, clinical presentation and results of other auxiliary tests, are necessary for the accurate diagnosis of myocarditis and can guide treatment accordingly. In the current case, a patient with viral myocarditis presented with retrosternal pain, elevated cardiac marker levels and ST-T changes on the ECG, similar to the clinical manifestations of acute myocardial infarction. Clinical manifestations, including a fever with temperature fluctuations and the appearance of a rash on the arms and legs, coronary angiography and the results of auxiliary examinations, could aid in the differential diagnosis between acute myocarditis and myocardial infarction. The etiology, pathology, diagnostics and therapy of myocarditis remain controversial. Future investigations are required to further unravel these questions.
The case report suggests that early glucocorticoid anti-inflammatory therapy and immunoglobulin therapy is of value to this type of patient.it is also important to note that in autopsy (not biopsy) report from China there is no inflammatory cell infiltrate but lots of endothelial sloughing.Abnormal coagulation is common in severe #COVID19 and D-Dimer > 1ug /dL is an independent risk factor for death.
Conclusions :
It is imperative to consider ischemic microvascular thrombosis therefore making use of anticoagulation almost mandatory.
References
- Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; (2017), Working Group on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 38:143–153. doi: 10.1093/ eurheartj/ ehw149.
- Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, Jernberg T. (2017), Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with non-obstructive coronary artery disease. Circulation.; 135:1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336.
- Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. (2015), Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 131:861–870. doi: 10.1161/CIRCULATIONAHA .114.011201.
- McCabe JM, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, Patel A, MacGregor JS, Hsue P, Stein JC, Kinlay S, Ganz P. (2012), Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch Intern Med.; 172:864–871. doi: 10.1001/ archinternmed.2012.945.
- Beltrame JF. (2013), Assessing patients with myocardial infarction and no obstructed coronary arteries (MINOCA). J Intern Med. 273:182–185. doi: 10.1111/j.1365-2796.2012.02591. x.
- Agewall S, Eurenius L, Hofman-Bang C, Malmqvist K, Frick M, Jernberg T, Tornvall P. (2011), Myocardial infarction with angiographically normal coronary arteries. Atherosclerosis.; 219:10–14. doi: 10.1016/j.atherosclerosis.2011.04.036.
- Niccoli G, Scalone G, Crea F. (2015), Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management.Eur Heart J. 36:475–481. doi: 10.1093/eurheartj/ wehu469.
- Arai AE. (2007), False positive or true positive troponin in patients presenting with chest pain but “normal” coronary arteries: lessons from cardiac MRI. Eur Heart J. 28:1175–1177. doi: 10.1093/eurheartj/ehl567.
- Pathik B, Raman B, Mohd Amin NH, Mahadavan D, Rajendran S, McGavigan AD, Grover S, Smith E, Mazhar J, Bridgman C, Ganesan AN, Selvanayagam JB. (2016), Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 17:1146–1152. doi: 10.1093/ ehjci / jev289
- Collste O, Sörensson P, Frick M, Agewall S, Daniel M, Henareh L, Ekenbäck C, Eurenius L, Guiron C, Jernberg T, Hofman-Bang C, Malmqvist K, Nagy E, Arheden H, Tornvall P. (2013), Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm Myocardial Infarction with Normal Coronaries study. J Intern Med. 273:189–196. doi: 10.1111/j.1365-2796.2012.02567.


