Archive : Article / Volume 1, Issue 1
- Review Article | DOI:
- https://doi.org/10.58489/2836-8851/003
Innovation in Neurosurgery: Lessons Learned, Obstacles, and Potential Funding Sources
- Rosalind Franklin University of Medicine and Science, Chicago, IL, USA.
- University of Nevada, Reno School of Medicine, Reno, NV, USA.
- University of Florida College of Medicine, Gainesville, FL, USA.
Brandon Lucke-Wold
Christopher B. Cutler, BS, Patrick King, BS, Majid Khan, BS, Bankole.O, BS, Brandon L.W, (2022). Innovation in Neurosurgery: Lessons Learned, Obstacles, and Potential Funding Sources. Neurons and Neurological Disorders. 1(2). DOI: 10.58489/2836-8851/003.
© 2022 Brandon Lucke-Wold, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 03-09-2022
- Accepted Date: 30-12-2022
- Published Date: 19-12-2022
neurosurgery, diagnosis, Pathology Analysis, Cardiology, Orthopedic.
Abstract
Innovation is central to neurosurgery and has dramatically increased over the last twenty years. Although the specialty innovates as a whole, only 3-4.7% of practicing neurosurgeons hold patents. Various roadblocks to innovation impede this process such as lack of understanding, increasing regulatory complexity, and lack of funding. Newly emerging technologies allow us to understand how to innovate and how to learn from other medical specialties. By further understanding the process of innovation, and the funding that supports it, Neurosurgery can continue to hold innovation as one of itsâs central tenets.
Introduction
Innovation has served as the backbone of modern Neurosurgery from the days of Cushing and Dandy and continues to drive Neurosurgery to greater heights and improved outcomes. With each advancement, diagnosis becomes more precise, surgery less invasive and recovery more prompt. Problems once heralded as insurmountable now are overcome daily no thanks to innovations. Despite the advancements made in the field, neurosurgery as a field thrives on innovation but still faces significant technological, financial and logistical barriers when it comes to innovation on a personal or institutional level. Although almost 100% of Neurosurgeons have an idea on how to improve a device or therapy, only 3-4.7 Percentile end up obtaining a patent on the idea [1,2]. One can logically conclude that even less bring those ideas to market. With the goal of increasing this number, this paper aims to detail the challenges, frameworks and funding available at all levels to help push innovation forward in Neurosurgery. Along the way we showcase innovations at the cutting edge of modern neurosurgery and highlight key lessons to be learned from innovations in other specialties. Our hope is to provide a resource for innovative neurosurgeons at all career stages and to spark discussion on the topic of innovation in this great field.
Current Innovations
Robotic Pedicle Screw Placement
Over the last decade, pedicle screw insertion in spine surgery has seen a great deal of advancement; beginning with manual-visual insertion (i.e., relying on preoperative radiographic imaging) to intra-operative computer-assisted navigation models, to more recent intraoperative robotic-assisted surgical applications [3-6]. Even more recent is the advent of augmented reality and machine learning applications for the use of pedicle screw insertion, including the use of 3D printed technologies [6-8]. Since their inception, these technology-driven advanced approaches have been increasingly adopted amongst neurosurgical and orthopedic fields [9]. These technologies are aimed at improving screw placement accuracy and procedure time while simultaneously decreasing invasiveness of surgery [7,10], further, studies have shown improved complication profiles of patients undergoing pedicle screw placement, especially with more recent advanced technology–including a lower risk-profile for surgeons performing these operations [7,9]. Notwithstanding increased costs associated with initial adoption of these advanced technologies, steep learning curve, and potential for technical malfunctions, pedicle screw insertion using these new technologies and approaches are becoming increasingly prevalent and show cost-effectiveness long-term due to less revision, lower infection rates, reduced length of stay, and shorter operation time [9,11].
Intraoperative Pathology Analysis
In neurosurgical oncology, the gold-standard for intraoperative histopathological identification is the use of fast-frozen sections to guide surgical decision making and postoperative care [12]. Tissue identification has long been an important part of neurosurgery since the days of Cushing [13]. Intraoperative histopathology allows the identification of tissue margins both for preservation of normal tissue and to ensure a gross total resection (GTR). Although the gold-standard, fast-frozen sections can require upwards of 30 minutes to complete [14,15], which is unpractical and inefficient when used to identify multiple areas for margins [16]. Other technologies have been developed to aid resection such as intraoperative MRI (iMRI) which is limited in its utility due to cost, macroscopic view and interruption to the surgical workflow [16,17]. While iMRI can help ensure GTR, it still requires histopathological identification. Other technologies such as fluorescence guided resection only work on enhancing tumors, limiting their use as many tumors are non-enhancing [16,18].
An ideal technology would marry rapid microscopic histopathological identification and real time margin analysis. Stimulated raman scattering histology (SRH) allows for real-time intraoperative microscopic imaging of unprocessed tissue [19]. Additionally, because the tissue is minimally processed, gross tissue architecture is preserved allowing for further insight into the tissue sample not possible with traditional pathology techniques [20]. Raman scattering microscopy (RSM) uses two laser sources to excite a tissue sample at various frequencies. The photons emitted generate a scattering pattern that can be detected and used to identify different tissues such as grey matter from glioma tissue [21,22]. Of note, this technology has also been used in a probe form, allowing for tissue identification at the probe point via spectroscopy (RSS) without removal of a sample [23]. Limitations include small sample area and unacceptable irradiation to brain tissue rendering it unsafe for clinical use at this time [24]. Pending advancements, RSS could find utility in needle guided procedures such as biopsy and DBS [25].
Although RSM has been around since 1999 [26], the technology has been limited by low sensitivity, fragile laser sources, low resolution and limited imaging speed rendering it unusable for intraoperative use [27]. Overcoming these limitations, Freudiger et al. developed a new tunable laser source and an all-fiber laser system with noise cancellation. These improvements allowed for high-speed imaging at speeds necessary for clinical utility [28]. In 2017, machine learning was used to color the images produced by SRM to match H&E staining in a technique called stimulated Raman histology (SRH) [20]. Further, SRH allows for the identification of non-glial tumors such as meningiomas and lymphomas [20]. This technology was first commercialized by Invenio-Imaging (Invenio Imaging Inc, Santa Clara, CA) (Figure 1) and is currently undergoing approval for use in the United States. Recently, the technology was shown how it can integrate into a typical neurosurgical operating room workflow [14,29-32]. We hypothesize that RSH will become a standard of neurosurgical oncology in the years to come given its limited disruption to surgical flow and increased utility over traditional pathological techniques

Simulated Raman Histology allows for rapid intraoperative pathology analysis with minimal disruption to the surgical workflow. A) tissue from the lesion or margin is resected and placed onto a slide (B). The slide is then inserted into the SRH machine to identify the tissue. C) the surgeon then uses the imaging from the machine to continue the resections allowing for complete margins in real time. Created with bioRENDER.com
WEB
A bifurcation aneurysm is a focal dilation of an artery at a point where one major vessel diverges into two. Wide-necked bifurcation aneurysms (WNBA) have been classically defined by a neck diameter greater than 4mm or a dome to neck ratio less than 2 [33]. However, research has shown that classification of wide necked bifurcation aneurysms via K-ratio, defined as [dome height+maximum dome width]/[2×maximum neck width] is more efficacious as it relates to decisions of management [34]. Vessels often affected by WNBA’s include the internal carotid artery, middle cerebral artery, anterior communicating artery, and the basilar artery [35].
Achieving intrasaccular flow disruption (IFD) while maintaining patency of the bifurcating vessel is a safe, low risk goal of treatment for WNBA [36]. A multitude of methods have been previously pursued to achieve this goal. Means such as stent assisted coiling and flow diversion have been used in setting of unruptured aneurysms, and balloon assisted coiling with Y-stenting and waffle cone technique have been used to treat both ruptured and unruptured aneurysms [37]. However, as aspects of these treatments such as stability of framework spanning aneurysmal neck and stability of coils inside the aneurysmal dome have been particularly challenging, attaining viable occlusion of aneurysm while preserving bifurcating vessel is a major challenge of treating WNBA [37-39]. It has been shown that patency of occlusion after one year is maintained in just half of patients treat with IFD [36]. Assessment of the Woven Endobridge Device (WEB) (Figure 2) have demonstrated that it is a safe and potent treatment for WNBA’s [38].
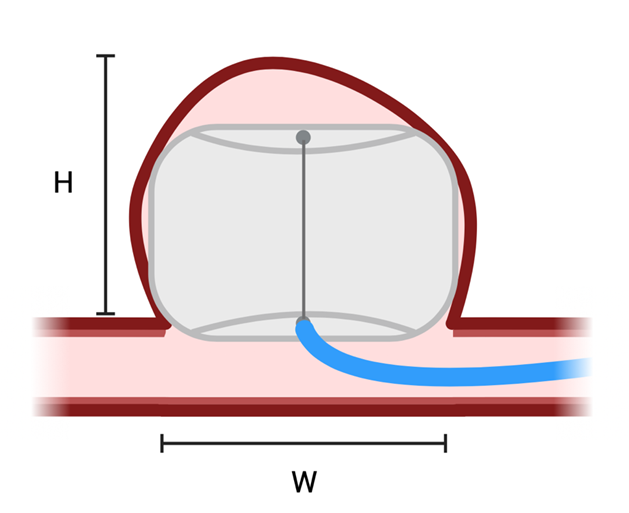
Figure 2: WEB Wide-necked bifurcation aneurysms (WNBA) have been classically defined by a neck diameter greater than 4mm (W) or a dome to neck ratio less than 2 [33]. However, research has shown that classification of wide necked bifurcation aneurysms via K-ratio, defined as [dome height (H)+maximum dome width (W)]/[2×maximum neck width] is more efficacious as it relates to decisions of management [34]. WEB is suited for these types of wide neck aneurysms. Created with bioRENDER.com
The WEB was developed by Sequent Medical Inc (acquired by Microvention in 2016) in California and initially introduced in Europe in 2011 before becoming FDA approved for use in the US in 2019 [39]. The WEB is an intrasaccular flow diverter that attains sturdy and comprehensive occlusion by establishing a stable framework that self-expands once inserted into the aneurysmal neck [35,39]. The original WEB (WEB-DL) was a cylindrical dual layer device composed of braided nitinol [35]. Newer versions of the WEB are single layered cylindrical (WEB-SL) spherically (WEB-SLS) devices [35]. As such, WEB is ideally suited for ovoid, cylindrical, and spherical aneurysms, but can also be used for irregularly shaped aneurysms when used to seal aneurysmal neck, taking advantage of the subsequent thrombosis of aneurysm [38]. Studies have shown long term safety and efficacy with use of WEB in WNBA as well as improved angiographic outcomes compared to conventional methods in subarachnoid hemorrhage [35,40].
Focused Ultrasound for Alzheimer’s
Focused ultrasound (FUS) is a non-invasive technique that uses multiple ultrasound emitting devices and directs their output to a targeted area in the brain to increase local temperature (Figure 3). When used simultaneously with intravenously injected microbubbles, the heat generated causes the microbubbles to expand and disrupt the local blood brain barrier [41]. FUS is being investigated for the treatment of multiple conditions either through local ablation or through disruption of the blood brain barrier to allow substances to pass through. One use of FUS currently under investigation is opening the blood brain barrier to allow passage of anti-amyloid and anti-tau antibodies, both endogenous and exogenous, to treat Alzheimer's Disease (AD) [42].

Figure 3: Focused Ultrasound. Sonication from the focused ultrasound device causes microbubble expansion and increased permeability of the blood brain barrier allowing large molecules (e.g. anti-amyloid antibodies) to pass through[141]. Created with bioRENDER.com
This technique has shown promise in animal models. Reduction in amyloid deposits and tau phosphorylation as well as behavioral changes including decreased anxiety and improved working memory have been reported in preclinical studies. Although there has been some success, there are still challenges to be overcome. The diffuse nature of brain damage in AD presents a unique optimization problem where maximizing the volume of blood brain barrier opening must be balanced against the risk of tissue damage. This optimization is complicated by the variability of different brain structures in their responsiveness to sonication [2]. Despite these challenges, the promising results in preclinical studies has spurred research in human subjects. As of yet, no significant adverse events have been reported and some have even found improvements in certain cognitive domains, though each were limited by small sample size and lack of long-term follow up [43-45]. There are larger phase I/II clinical trials currently underway that should provide more reliable insights [42,46-50].
Frameworks and barriers of Innovation in neurosurgery
Although medicine has advanced rapidly, and will continue to advance in the years to come, there are significant barriers that must be overcome to innovate and drive medicine forward. Each year the FDA receives around 22,000 applications for new products with only 7-9% eventually reaching the market [51]. However, according to the FDA, each year more novel products are approved with 132 being approved in 2020 alone indicating that innovation continues to grow [52,53]. Despite this growth, various barriers have to be overcome to successfully innovate in medicine. One systematic review by Warty et al. found that these barriers include regulation, technological challenges, lack of clinical evidence, health technology assessment, reimbursement, and adoption51. This supported what Bergsland et al found in 2014 [54]. Each of these barriers have to be overcome for a device to make it to market. On average, it takes around 17 years for a device to reach the market indicating a substantial investment in both time and money [55]. In contrast, Elliott et al. looked at innovation barriers from the perspective of physicians and found that legal and regulatory issues tended to curb innovation [56]. For device innovation, there are two regulatory pathways: de novo or 510(k) that guide this process in the US. In 510(k) premarket approval, devices are streamlined through the FDA by demonstrating that the device is substantially similar to another device (a predicate) already on the market [57]. Most devices approved through the 510(k) process have on average 1-2 predicates [58]. By using a predicate, products can be brought to market more rapidly than through the de novo approval pre-market FDA process.
Neurosurgery shares these barriers with the rest of medicine. Neurosurgical innovation has been qualitatively as well as quantitatively assessed recently [1,59,60]. These papers found that although neurosurgery as a field has been innovative, innovation is institutionally lead and driven by a handful of neurosurgeons. Babu et al found that only 3% (n=147) of practicing neurosurgeons held 582 patents between 1976-2012 [1]. This number has increased to 4.7% as of 2020 [60].
Various frameworks have been elucidated to facilitate medical device innovation [51,61-63]. These frameworks are designed to prevent “the innovation from becoming an answer in search of a problem”[64]. The IDEAL framework was first proposed in 2009 by McCulloch et al and augmented the traditional pharmacological trial framework to include steps specific to devices and surgical techniques [62] (Figure 4). Although frameworks such as IDEAL have been beneficial in guiding innovation, they are not without critique[65-67]. Muskens et al. analyzed several innovations brought to market using the IDEAL framework and found that they did not follow all the recommended steps, thus calling the utility of the framework into play [67]. Ota et al. reviewed neurosurgical innovation using IDEAL and found that unlike other surgical subspecialities, neurosurgery has been slow to adopt the framework perhaps due to unique limitations of the specialty[68]. Recently, the IDEAL framework has been adapted to meet some of these critiques[61,69]. Regardless of the framework chosen, these processes will be further refined to improve patient safety and increase the rate of innovation in neurosurgery in the years to come.

Figure 4: IDEAL framework for surgical innovation [62]. Stage 0 was later added to mirror the pharmaceutical industry’s policy to become the IDEAL-D framework [61]. Created with bioRENDER.com
Innovation in other medical specialties
Innovation in medicine goes beyond the borders of neurosurgery. Fields such as cardiology and orthopedic surgery as well as the industry responsible for the biomedical devices used across healthcare are spaces where advancements are constant. Growth in each of these settings can process by which these breakthroughs are made.
Biomedical
Medical devices are an integral part of medicine that have a major influence on the overall landscape of healthcare as well as the day-to-day activities withing the healthcare space. Today we regularly use some of the most significant advances in biomedical device development, including inventions such as X-Ray, CT, MRI, pacemakers, and the pulse oximeter [70]. Advancements in the arena have been and continue to be crucial for advancement of medicine in the paradigms of patient care, outcomes, access, and beyond. However, medical device innovation is a laborious process with many layers and facets including identifying a need, verifying its distinction, synthesizing reasonable solutions, testing, and roll out to an appropriate market [71]. Each of these aspects must be carefully thought about in order to avoid the development of a product that is the solution to a nonexistent problem [2]. This is a process that can take up to 10 years and carries risks and challenges that should be weighed before and during undertaking [71,72].
Despite the challenges to the process of biomedical innovation, modern strides are still being made. Once such advancement is the advent of the three-dimensional (3D) printing of biomaterial. Beginning with synthesis of larger substances such as dental implants and prosthetics, 3D printing has evolved to now include the assembly of material composed of active biomolecules and living cells [73]. This has opened the door for its use in in-vitro applications such as disease modeling and drug screening [73]. Another recent significant advancement is in the realm of genetics with the advent of the Clustered Regularly-Interspaced Short Palindromic Repeats (CRISPR) which promises application across many areas of research and medicine including gene editing, transcriptional modification, and epigenetic targeting.
As the world recovers from the COVID-19 pandemic, greater attention in many fields, including device development, is being given to simplifying remote delivery of healthcare. Two areas of concentration for this movement include advancements in devices that facilitate remote monitoring and Artificial Intelligence (AI). Research has investigated the remote gathering of patient information from wearable and implanted devices in the care of heart failure patients [74,75]. AI has the potential to revolutionize almost every field of medicine [76]. Studies have investigated the use of AI in anesthesiology, medical imaging, drug design, cardiology and more [77-80].
Cardiology
Cardiology is a major leader in the space of AI in medicine, with recent advancements being made in risk prediction, signal processing, and analysis of imaging, ECG’s, and large data. In addition to AI, cardiology also sits at the forefront of medical innovations in other ways. The history of innovation in cardiology is a rich one, with many discoveries spanning the centuries. The most significant of these include the advent of the ECG in the 19th century, cardiac catheterization in the 1920’s, and the Echocardiogram and Automatic Implantable Cardioverter-Defibrillator (AICD) in the 1950’s.
Because of its clinical range as well as it history, initiatives in innovation have used cardiology as a paradigm of investigation and advancement, guided by a process of Identification of needs, invention of solutions, and implementation; similar to what takes place in the biomedical device space [81].
One of the more recent notable products of innovation in cardiology is the left ventricular assist devices (LVAD) used for treatment of refractory heart failure. This device was pioneered to address the shortage of organs accessible for heart transplant82. The LVAD has been shown to improve survival and quality of life in patients when compared to medical therapy [82].
As radiation exposure engendered by cardiologist is an issue being discussed, robotic technology is being investigated as a potential solution to reduce harm [83,84]. AI, robotics, and the process of innovation are all advances that have the potential to transcend cardiology and make their way into neurosurgery.
Ortho
Orthopedic surgery is another field where innovation plays a key role in everyday practice. Robotics has been in orthopedics for a while and has thus far shown increased precision with decreased need for revision as well as improved patient satisfaction [85]. With looming concerns of cost and time, the established track record of robotics increasing efficiency across other fields holds promise [85]. 3D printing is also being used to improve patient care with products assisting in preoperative planning as well as personalized implants and splints [86].
The logistics of orthopedic practice, however make the progression of identification to invention and implementation a formidable task that is often laborious to overcome [87]. As such, it has been proposed that these challenges might be quelled with early collaboration between surgeons, researchers, and companies to allow for adequate support for ideas [88]. As lack of familiarity with the process of product development contributes to this challenge as well, it has also been proposed that aspiring innovators can capitalize on the networks constructed by orthopedic societies to propel progress [89]. This means of solution to the logistical barriers of innovation can be applied to the field of neurosurgery.
As innovation is integral to the progression of neurosurgery, understand the approaches to innovation across other fields can serve as a means to assimilating processes, anticipation obstacles and learning from solutions other fields have used to address their challenges.
How innovation could be advanced in neurosurgery
Neurosurgery is an especially technologically advanced discipline which is constantly developing over time; uniquely, the establishment of surgery on the brain and CNS followed breakthroughs in microbiology and anesthesia, and understandings of ventricle and cerebral vascular anatomy, and head trauma [90,91]. The culmination of advancements in neuroscience, surgery, and technology have allowed for significant breakthroughs in neurosurgery (e.g., DBS, awake craniotomy, focused ultrasound, etc.);[92-94] further, sub-specialization and involvement of multidisciplinary teams have allowed for even more advancements in a variety of neurosurgery fields (e.g., neuro-oncology, neuro-IR, pediatric and functional neurosurgery) [95-98]. These advancements began from the ground up, often due to one individual – this highlights the continued importance of innovations in neurosurgery at all levels.
At the medical school level, innovation can be introduced and nurtured through the development of student interest groups, meetings with local neurosurgeons, and further, involvement at a national level with established neurosurgery societies (e.g., AANS and CNS). One recent example is the establishment of innovation and entrepreneurship training program at Washington University School of Medicine (St. Louis), which foster collaboration between medical and engineering teams and yield the generation of new ideas and could be the beginning to allowing more breakthroughs in the field [99]. Medical schools as a whole can promote and incorporate an innovation-led curriculum (i.e., lecture series and hands-on modules with completion of a capstone project), however, less than 20% of American and Canadian medical schools have or participate in this type of curriculum [100]. Generating a longitudinal interdisciplinary elective may also aid in this effort; for instance, Stony Brook University School of Medicine incorporated an initiative of a novel 3-year biodesign course for medical students, which resulted in fostering opportunities for seminars, group projects, clinical experience discussions, concept mapping and product development, as well as reflection and mentoring [101]. Furthermore, incorporating entrepreneurship curriculum and opportunities at this early career stage may foster future advancements in the field and promote openness in sharing ideas as trainees will be more acquainted with the landscape of innovation – ultimately generating lifetime problem solvers [102]. There are national programs in which college and graduate students come together in an inventors competition, which doubles as a networking event with world-class inventors from across the world; [103] further, neurosurgical organization (e.g., CNS) host innovator of the year competitions which fosters year-round activity in advancing the field [104]. In lieu of organizational support, one can follow a novel funding strategy in which individuals can start a crowdfunding campaign; uniquely, the campaigns can be done online and through social media, effectively doubling as a method to raise awareness of a particular clinical problem [105]. The added benefit of medical students participating in such activities is that they help bolster their applications into residency, offering programs insight into their commitment and interest to neurosurgical training. The reality of coming together as a community revolving around innovation is that the field will further see an increase in the rate of innovation and allow for better and novel treatments for neurosurgical pathologies.
At the residency level, there are many ways innovation can be fostered. This can begin at residency programs with incorporation of surgical innovation and entrepreneurship discussions; further, the addition of seminars and conferences led by faculty or pioneers in the field can also foster new ideas and allow for immediate implementation and analysis of these innovations in the clinical care setting [106,107]. One example of this is, though in Ophthalmic surgery, was the incorporation of a program for faculty and trainees to identify, innovate, and implement clinically meaningful change; ultimately, they generated 19 novel proposals for improvement of the department.[108]. One of these proposals led to improved OR utilization and reduction in cancelled/rescheduled surgeries, which ultimately increased their positive financial margin by $141k, all while maintaining patient care and increasing patient, surgeon and staff satisfaction [108]. The philosophy that at one point in time, someone or something challenged current standard of care practices in neurosurgery, which led to the improvement of the field is one all neurosurgeons should embody. In particular, the generation of medical devices has pushed the boundaries of what we are able to achieve in the treatment of patients in a safer and more efficient manner. In one neurosurgical program, the establishment of a center of innovation has fostered the generation of many novel device innovations through several partnerships with neurosurgeons, patent law students, and biomedical engineering students; importantly, this effort was supported with modest start-up capital funding and strong faculty and institutional support [109]. In under two years, the program was able to expand nationwide and allowed for many multidisciplinary teams to give their input and feedback, generating substantial intellectual property, educational opportunities, and business ventures [109]. It may be easier for neurosurgery trainees and senior neurosurgeons to identify areas in need of innovation as they are at the forefront of patient care in neurosurgery – and, it may be a good idea to get the opinions of other healthcare teams in order to get a diverse understanding of potential problems and innovative solutions. The success of these initiatives illustrates the potential of implementing these programs throughout the country. Innovation in neurosurgery might benefit from neurosurgical faculty involvement and could be bolstered by collaboration with business and engineering programs.
Resources available for innovating in NSGY
There are a variety of funding mechanisms that exist for both research and innovation in neurosurgery (Table1).
Tables:
Table 1. Funding Mechanisms for Innovation in Neurosurgery
Trainee Level | Agency | Name | Funding Amount | Timeline | Dual-Degree (MD/PhD) | Ref |
Predoctoral (Medical student) | NIH | Individual Predoctoral National Research Service Awards For M.D./Ph.D. Fellowships (F30) | Up to $126k | Up to 6 years | Yes | 110 |
|
| Predoctoral Fellowship Awards to Promote Diversity in Health-Related Research (F31) | Up to $126k | Up to 6 years | Yes | 110 |
|
| Individual Predoctoral National Research Service Awards For Individual Predoctoral Fellows (F31) | Up to $126k | Up to 6 years | Yes | 110 |
|
| Medical Research Scholars Program (Previously Medical Student Scholars Program - F31) | $41,000+ | 1 year program |
| 110 |
| NIH & NIGMS Medical Scientist Training Program (MSTP) | NIH Medical Scientist Training Program (MSTP) | Varies | Up to 5 years Certain medical schools in the US | Yes | 110 |
| Neurosurgery Research & Education Foundation (NREF) | Medical Student Summer Research Fellowship | $2,500 | Up to 4 months |
| 131 |
| AANS | MISSION Fellowship | $1,000 | 2 years |
| 111 |
| CNS & CSNS | Medical Student Summer Fellowship in Socioeconomic Research | $2,500 | 8-10 weeks |
| 129 |
| Department of Defense (DoD) | National Defense Science and Engineering Graduate Fellowship | $121,400+ | Up to 3 years |
| 110 |
|
| Congressionally Directed Medical Research Programs | Up to $5m | Up to 3 years |
| 110 |
| Doris Duke Charitable Foundation | Clinical Research Fellowship for Medical Students | Varies | Certain medical schools in the US |
| 110 |
| Howard Hughes Medical Institute (HHMI) | Research Training Fellowships for Medical Students | $38,000 | 1 year |
| 110 |
| Howard Hughes Medical Institute (HHMI) & NIH | National Institutes of Health (NIH) Research Scholars Program (NIH Cloister Program) | $27,000+ | 1 year at the NIH campus |
| 110 |
| Sarnoff | Sarnoff Fellowship Program | $34,500+ | 1 year |
| 110 |
| AMA | Foundation Seed Grant Research Program | $2,500 |
|
| 110 |
| Glenn/AFAR | Student Scholarships for Research in the Biology of Aging | Varies | Up to 6 months |
| 110 |
| Hospitals | Hospital Seed Grants | Varies | Varies |
|
|
Postdoctoral (Resident/Fellow) | NIH | Individual Postdoctoral National Research Service Awards For Individual Postdoctoral Fellows (F32) | $330,000+ | Up to 6 years |
| 110,130 |
|
| Mentored Research Scientist Career Development Award (K01) | $575,000+ | Up to 5 years |
| 130 |
|
| Academic Career Development Award (K07) | $625,000+ | Up to 5 years |
| 130 |
|
| Mentored Clinical Scientist Research Career Development Award (K08) | $250,000+ | Up to 5 years |
| 130 |
|
| Career Transition Award (K22) | $450,000+ | Up to 3 years |
| 130 |
|
| Mentored Patient-Oriented Research Career Development Award (K23) | $750,000+ | Up to 5 years |
| 130 |
|
| Mentored Quantitative Research Career Development Award (K25) | $750,000+ | Up to 5 years |
| 130 |
|
| Emerging Global Leader Award (K43) | $525,000+ | Up to 5 years |
| 130 |
|
| Emerging Leaders Career Development Award (K76) | $750,000+ | Up to 5 years |
| 130 |
|
| Pathway to Independence Award (K/99/R00) | $1m+ | Up to 5 years |
| 130 |
| Neurosurgery Research & Education Foundation (NREF) | Research Fellowship Grant | $50,000 | 1 year |
| 131 |
|
| Young Clinician Investigator Awards | $50,000 | 1 year |
| 131 |
|
| Clinical Fellowship Grant | $75,000 | Varies |
| 111,131 |
| AANS & NREF | William P. Van Wagenen Fellowship | $135,000+ | Up to 1 year |
| 111,131 |
| The Damon Runyon Cancer Research Foundation | Damon Runyon Fellowship Award | $270,000 | 4 years |
| 110 |
| CNS & The National Institute of Neurological Disorders and Stroke (NINDS)
| Research Education Grant (R25) Programs for Residents and Fellows in Neurology, Neurosurgery, Neuropathology, and Neuroradiology | $140,000+ | Up to 2 years |
| 112 |
Commercialization (Any level) | NSF | American Seed Program | Up to $2m | / |
| 116 |
|
| ICORPS – NRF | Varies | / |
| 117 |
| NIH | SBIR & STTR programs | Up to $1.8m | / |
| 132 |
|
| C3i | Varies | / |
| 118 |
|
| REACH | Varies | / |
| 119 |
|
| NIGMS – Fellowships | Varies | / |
| 120 |
In addition, there are several public and private funding organizations/programs which offer financial support to trainees who which to translate their findings into an actionable product or campaign. Focusing on funding opportunities, trainees in medical school can apply for funding through Predoctoral fellowships. If the medical student is an MD/PhD student, they can apply for National Institutes of Health (NIH) F30 and F31 fellowship awards, which provides support throughout their medical and graduate career [110]. For non-dual-degree medical trainees (including residents), there are also a number of pre- and post-doctoral fellowship opportunities offered through nationally recognized communities (e.g., NIH, Congress of Neurological Surgeons [CNS], American Medical Association [AMA], American Association of Neurological Surgeons [AANS], Neurosurgery Research & Education Foundation [NREF]) [110-114]. Other mechanisms for non-dual-degree medical students are research career development awards (i.e., NIH K awards), which provide trainees with mentored and independent research scientist awards to foster research skills and experience in fundamental sciences dependent on trainee level [115]. In addition, many hospitals and neurosurgery residency programs offer their own innovation funding resources.
Depending on trainee level or progression of novel innovation, commercialization resources exist to take these innovations to a broader audience and application. The National Science Foundation (NSF) provides an American seed program, in which an individual can obtain up to $2 million in seed grants for 0% equity. The prerequisite for this type of funding/support is that the innovative idea is something already research and established, rather than to obtain preliminary data on its effectiveness [116]. The NSF also has a program which fosters a training program for scientists and engineers, doubling as an outlet to foster partnerships between regional universities and industry (i.e., Innovation-corps) [117]. They have over $750 million in total funding, and over a hundred startups have formed through this pathway alone [117]. The NIH has two programs which allow small business innovation research and technology transfer (e.g., The Small Business Innovation Research [SBIR] and Small Business Technology Transfer [STTR] programs). These programs provide non-dilutive funding and special funding for research topics. Furthermore, the NIH offers education programs to help investigators translate their research into marketable products (e.g., The Concept to Clinic: Commercializing Innovation [C3i]) program and Research Evaluation and Commercialization Hubs [REACH]) [118,119]. Specially, the NIH also recognizes that certain states have historically low funding, and offer other seed initiatives to foster growth of ideas in these states (e.g., via the National Institute of General Medical Sciences [NIGMS]) [120].
Discussion
Innovation is and has been central to Neurosurgery. In the past 20 years we have seen the development of novel instruments, robotics, imaging techniques and devices, and vascular devices that are all now central to how Neurosurgery is practiced. Between 2009 and 2014, medical device patents across all of medicine grew by 140% [121]. With innovation being so central to innovation in Neurosurgery, and the rapid growth in device patents over the past decade, why is it that only 3-4.7% of practicing neurosurgeons obtain a device patent [1,2]? There may be several reasons for this. Although the number of device patents has exponentially increased over the last 20 years, physician involvement in device patents has remained relatively stagnant [121]. This discrepancy could be explained by the increasing cost and complexity of bring a device to market [122]. As complexity increases, more and more innovation is led by industry often with physicians in a consulting role. The low number of patents issued in Neurosurgery does not reflect such a role that a neurosurgeon can play in device innovation. Further, not all innovation requires a patent [123]. Other times a company might refrain from obtaining a patent to guard trade secrets. This would further reduce the percentage. One way to combat the stagnation of physician involvement is to foster interest and understanding of device development throughout medical education. As mentioned earlier, only 20% of US and Canadian allopathic medical schools currently offer any curriculum centered around innovation although this number has been increasing year over year[100]. 57% of schools offering such programs started in the last four years [100]. Proposed improvements to medical school innovation curricula include increasing involvement of industry partners and an interprofessional approach between medical students and professions such as engineering, business and law[102]. Others have proposed that innovation should be a core tenet taught in medical school[124]. Beyond medical school, very few post graduate programs exist for medical device innovation[109,125,126]. We predict that these programs will increase in number and popularity in the years to come as innovation becomes even more central to medicine and specifically neurosurgery. Despite all these limitations, neurosurgery was the specialty with the highest percentage of patent holders between 1993 and 2018 [121], highlighting the strong history of innovation in neurosurgery.
Many innovations can be very costly to develop. To the best of our knowledge, there are not published studies on the cost to bring a device to market. However, a 2010 industry survey estimated the cost to be between $31 million and $94 million depending on the approval pathway used [127]. A more recent 2020 white paper estimated the funds raised for device development to be $25.5 million with about $2 to $5 million going towards engineering and development [128]. These costs can present a significant hurdle to innovation, especially for neurosurgeons that do not have the resources of a large biomedical device company. In this paper, we covered over 30 available sources of funding to help overcome this hurdle (Table1). Extensive funding opportunities exist for basic research and discovery at all career stages [110-112,129-131]. Efforts that leverage these resources can serve as kernels for innovative solutions which can then be scaled up using commercialization funds from the NSF and NIH [116-120,132]. This pipeline represents a significant resource available to innovative neurosurgeons, facilitating the process from inception to implementation. Despite the numerous sources available, no clear data exists in aggregate on the overall utilization of these funds. We believe that awareness can only increase their usage. Our hope is that this paper can direct increased use of these funds at all levels, spurring innovation in the process.
Given the complexity of device innovation, collaboration with industry is almost a prerequisite to succeed in today’s market. With these collaborations there as been an increasing call for improved transparency with these relationships. With the advent of the sunshine act in 2009, congress sought to bring to light physician industry payments [133]. Since the creation of this act, many have claimed that mandatory reporting of industry payments is not enough to ensure total transparency. Specialties such as Orthopedics and Spine have come under considerable light as to the impact payments have on physician trust [133]. Some have gone as far as suggesting that no industry relationship should be maintained by practicing physicians [134]. However, in Neurosurgery patient experience and trust has not been shown to be affected by industry payments [135]. This has been in spite of neurosurgery having the second highest average total payment per physician of any specialty [136]. Further innovation should always factor in industry relationships and the impact they might have on patient care and trust as we continue to technologically advance neurosurgery.
Innovation has always been a part of neurosurgery. Widespread adoption of new technologies will take time but are posed to radically change the way neurosurgery is practiced. Robotics were once novel but are now reported to be used by 51% of neurosurgeons practicing in the US [137]. Other technologies critical to modern neurosurgery like the operative microscope are posed to be replaced. Although operative microscopes form a part of most neurosurgical operating rooms, 5.8% of neurosurgeons have switched to the use of exoscopes for intraoperative maginification [138,139]. Furthermore, the use of these newer innovations are associated with a higher perception of neurosurgical training according to residents[140]. As we ride this wave of innovation, we can improve patient outcomes, streamline diagnosis and treatment and minimize physician burnout.
Conclusion
Neurosurgery has been, and will continue to be, a highly innovative field. Through learning from successes in other innovative specialties as well as through awareness of the barriers to, resources for, and frameworks of innovation, this tendency can be made even stronger. It is our hope that the information presented here can serve to inspire and facilitate further innovation in this great field.
References
- Babu MA, Heary RF, Nahed BV. Device innovation in neurosurgery: controversy, learning, and future directions. Neurosurgery. 2012;70(4):789-795.
- Baron RB, Kessler RA, Bhammar A, et al. Patents and Innovation Among Neurosurgeons from the American Association of Neurological Surgeons. Cureus. 2020;12(2):e7031.
- Verma R, Krishan S, Haendlmayer K, Mohsen A. Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J. 2010;19(3):370-375.
- Helm PA, Teichman R, Hartmann SL, Simon D. Spinal Navigation and Imaging: History, Trends, and Future. IEEE Trans Med Imaging. 2015;34(8):1738-1746.
- Perfetti DC, Kisinde S, Rogers-LaVanne MP, Satin AM, Lieberman IH. Robotic Spine Surgery: Past, Present, and Future. Spine (Phila Pa 1976). 2022;47(13):909-921.
- Liu Y, Lee MG, Kim JS. Spine Surgery Assisted by Augmented Reality: Where Have We Been? Yonsei Med J. 2022;63(4):305-316.
- Zhou LP, Zhang RJ, Sun YW, Zhang L, Shen CL. Accuracy of Pedicle Screw Placement and Four Other Clinical Outcomes of Robotic Guidance Technique versus Computer-Assisted Navigation in Thoracolumbar Surgery: A Meta-Analysis. World Neurosurg. 2021;146:e139-e150.
- Yu C, Ou Y, Xie C, Zhang Y, Wei J, Mu X. Pedicle screw placement in spinal neurosurgery using a 3D-printed drill guide template: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15(1):1.
- Lopez IB, Benzakour A, Mavrogenis A, Benzakour T, Ahmad A, Lemee JM. Robotics in spine surgery: systematic review of literature. Int Orthop. 2022.
- Carazzo CA, Yurac R, Guiroy A, et al. Minimally Invasive Versus Open Surgery for the Treatment of Types B and C Thoracolumbar Injuries: A PRISMA Systematic Review. Int J Spine Surg. 2021;15(4):803-810.
- Soliman MAR, Pollina J, Poelstra K, Chaudhary S, Foley K. Can a Spine Robot Be More Efficient and Less Expensive While Maintaining Accuracy? Int J Spine Surg. 2022.
- Straehle J, Erny D, Neidert N, et al. Neuropathological interpretation of stimulated Raman histology images of brain and spine tumors: part B. Neurosurgical review. 2022;45(2):1721-1729.
- Eisenhardt L, Cushing H. Diagnosis of intracranial tumors by supravital technique. The American Journal of Pathology. 1930;6(5):541.
- Di L, Eichberg DG, Huang K, et al. Stimulated Raman histology for rapid intraoperative diagnosis of gliomas. World neurosurgery. 2021;150:e135-e143.
- Novis DA, Zarbo RJ. Interinstitutional comparison of frozen section turnaround time. Archives of pathology & laboratory medicine. 1997;121(6):559.
- Pekmezci M, Morshed RA, Chunduru P, et al. Detection of glioma infiltration at the tumor margin using quantitative stimulated Raman scattering histology. Scientific reports. 2021;11(1):1-11.
- Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. The lancet oncology. 2011;12(11):997-1003.
- Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of maximal extent of resection of contrast-enhanced and non–contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA oncology. 2020;6(4):495-503.
- Broadbent B, Tseng J, Kast R, et al. Shining light on neurosurgery diagnostics using Raman spectroscopy. Journal of neuro-oncology. 2016;130(1):1-9.
- Orringer DA, Pandian B, Niknafs YS, et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nature biomedical engineering. 2017;1(2):1-13.
- Kalkanis SN, Kast RE, Rosenblum ML, et al. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. Journal of neuro-oncology. 2014;116(3):477-485.
- Zhang J, Fan Y, He M, et al. Accuracy of Raman spectroscopy in differentiating brain tumor from normal brain tissue. Oncotarget. 2017;8(22):36824.
- Desroches J, Jermyn M, Pinto M, et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Scientific reports. 2018;8(1):1-10.
- DePaoli D, Lemoine É, Ember K, et al. Rise of Raman spectroscopy in neurosurgery: a review. Journal of Biomedical Optics. 2020;25(5):050901.
- DePaoli DT, Lapointe N, Messaddeq Y, Parent M, Côté DC. Intact primate brain tissue identification using a completely fibered coherent Raman spectroscopy system. Neurophotonics. 2018;5(3):035005.
- Zumbusch A, Holtom GR, Xie XS. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Physical review letters. 1999;82(20):4142.
- Freudiger CW, Min W, Saar BG, et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 2008;322(5909):1857-1861.
- Freudiger CW, Yang W, Holtom GR, Peyghambarian N, Xie XS, Kieu KQ. Stimulated Raman scattering microscopy with a robust fibre laser source. Nature photonics. 2014;8(2):153-159.
- Hollon TC, Lewis S, Pandian B, et al. Rapid intraoperative diagnosis of pediatric brain tumors using stimulated Raman histology. Cancer research. 2018;78(1):278-289.
- Hollon TC, Pandian B, Adapa AR, et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nature medicine. 2020;26(1):52-58.
- Jiang C, Bhattacharya A, Linzey JR, et al. Rapid Automated Analysis of Skull Base Tumor Specimens Using Intraoperative Optical Imaging and Artificial Intelligence. Neurosurgery. 2022;90(6):758-767.
- Neidert N, Straehle J, Erny D, et al. Stimulated Raman histology in the neurosurgical workflow of a major European neurosurgical center—part A. Neurosurgical Review. 2022;45(2):1731-1739.
- Lazareska M, Aliji V, Stojovska-Jovanovska E, et al. Endovascular treatment of wide neck aneurysms. Open access Macedonian journal of medical sciences. 2018;6(12):2316.
- Park HS, Kwon SC, Park ES, Park JB, Kim MS. A new definition for wide-necked cerebral aneurysms. Journal of Cerebrovascular and Endovascular Neurosurgery. 2019;21(4):193-198.
- Fujimoto M, Lylyk I, Bleise C, Albiña P, Chudyk J, Lylyk P. Long-term outcomes of the WEB device for treatment of wide-neck bifurcation aneurysms. American Journal of Neuroradiology. 2020;41(6):1031-1036.
- Chen C-J, Dabhi N, Snyder MH, et al. Intrasaccular flow disruption for brain aneurysms: a systematic review of long-term outcomes. Journal of Neurosurgery. 2021;1(aop):1-13.
- Pierot L, Biondi A. Endovascular techniques for the management of wide-neck intracranial bifurcation aneurysms: a critical review of the literature. Journal of Neuroradiology. 2016;43(3):167-175.
- Goyal N, Hoit D, DiNitto J, et al. How to WEB: a practical review of methodology for the use of the Woven EndoBridge. Journal of neurointerventional surgery. 2020;12(5):512-520.
- Muskens IS, Senders JT, Dasenbrock HH, Smith TR, Broekman ML. The Woven Endobridge device for treatment of intracranial aneurysms: a systematic review. World Neurosurgery. 2017;98:809-817. e801.
- Pennig L, Goertz L, Hoyer UCI, et al. The Woven EndoBridge (WEB) versus conventional coiling for treatment of patients with aneurysmal subarachnoid hemorrhage: propensity score-matched analysis of clinical and angiographic outcome data. World Neurosurgery. 2021;146:e1326-e1334.
- Fishman PS, Frenkel V. Focused ultrasound: an emerging therapeutic modality for neurologic disease. Neurotherapeutics. 2017;14(2):393-404.
- Souza RMdC, Silva ICSd, Delgado ABT, Silva PHVd, Costa VRX. ULTRASSONOGRAFIA FOCALIZADA E DOENÇA DE ALZHEIMER: UMA REVISÃO SISTEMÁTICA. Dementia & Neuropsychologia. 2018;12:353-359.
- Jeong H, Song I-U, Chung Y-A, et al. Short-Term Efficacy of Transcranial Focused Ultrasound to the Hippocampus in Alzheimer’s Disease: A Preliminary Study. Journal of Personalized Medicine. 2022;12(2):250.
- Lipsman N, Meng Y, Bethune A, et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 9 (1): 2336. In:2018.
- Mehta RI, Carpenter JS, Haut M, et al. Blood-Brain Barrier Opening with MRI-guided Focused Ultrasound Elicits Meningeal Venous Permeability in Humans with Early Alzheimer Disease. Radiology. 2021;298(3):654-662.
- InSightec. A Phase IIa Study to Evaluate the Safety and Efficacy of Blood-Brain Barrier (BBB) Opening Using Transcranial MR-Guided Focused Ultrasound in Patients With Alzheimer’s Disease. 2021;
- InSightec. Assessment of Safety and Efficacy of ExAblate Blood-Brain Barrier Disruption for the Treatment of Patients With Probable Alzheimer’s Disease. 2022;
- Angeles NAoWL. The Use of Transcranial Focused Ultrasound for the Treatment of Neurodegenerative Dementias. 2021; https://clinicaltrials.gov/ct2/show/NCT04250376. Accessed JUly 17, 2022.
- Konofagou E. Neuronavigation-Guided Focused Ultrasound-Induced Blood-Brain Barrier Opening in Alzheimer’s Disease Patients. 2022;
- Kuhn T. Study of Non-Invasive Deep Brain Stimulation With Low Intensity Focused Ultrasound Pulse (LIFUP) for Mild Cognitive Impairment (MCI) and Mild Alzheimer’s Disease (AD). 2020.
- Warty RR, Smith V, Salih M, Fox D, McArthur SL, Mol BW. Barriers to the diffusion of medical technologies within healthcare: A systematic review. IEEE Access. 2021.
- Progress in Achieving Our Vision of Patients First. In. Vol 2021. US Food and Drug Administration: US Food and Drug Administration.
- Reflections on a Record Year for Novel Device Innovation Despite COVID-19 Challenges [press release]
- Bergsland J, Elle OJ, Fosse E. Barriers to medical device innovation. Medical devices (Auckland, NZ). 2014;7:205.
- Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510-520.
- Elliott T, Miola J, Samanta A, Samanta J. Fears and fallacies: Doctors’ perceptions of the barriers to medical innovation. Clinical Ethics. 2019;14(4):155-164.
- How to Find and Effectively Use Predicate Devices. 2018. https://www.fda.gov/medical-devices/premarket-notification-510k/how-find-and-effectively-use-predicate-devices. Accessed 07/08/2022.
- Pai DB. Mapping the genealogy of medical device predicates in the United States. Plos one. 2021;16(10):e0258153.
- Marcus HJ, Hughes-Hallett A, Kwasnicki RM, Darzi A, Yang G-Z, Nandi D. Technological innovation in neurosurgery: a quantitative study. Journal of neurosurgery. 2015;123(1):174-181.
- Motiwala M, Kumar R, Ajmera S, et al. Innovation, royalties, and introduction of the patent Hirsch index within US Academic neurosurgery. World Neurosurgery. 2020;137:e395-e405.
- Marcus HJ, Bennett A, Chari A, et al. IDEAL-D Framework for Device Innovation: A Consensus Statement on the Preclinical Stage. Ann Surg. 2022;275(1):73-79.
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105-1112.
- Pennell CP, Hirst A, Sedrakyan A, McCulloch PG. Adapting the IDEAL framework and recommendations for medical device evaluation: a modified Delphi survey. International Journal of Surgery. 2016;28:141-148.
- Ammirati M. Technological innovation and neurosurgery. Acta Neurochirurgica. 2021;163(8):2093-2093.
- Aylmore H, Dimitrakakis E, Carmichael J, et al. Specialised Surgical Instruments for Endoscopic and Endoscope-Assisted Neurosurgery: A Systematic Review of Safety, Efficacy and Usability. Cancers. 2022;14(12):2931.
- Macefield RC, Wilson N, Hoffmann C, et al. Outcome selection, measurement and reporting for new surgical procedures and devices: a systematic review of IDEAL/IDEAL-D studies to inform development of a core outcome set. BJS open. 2020;4(6):1072-1083.
- Muskens I, Diederen S, Senders J, et al. Innovation in neurosurgery: less than IDEAL? A systematic review. Acta neurochirurgica. 2017;159(10):1957-1966.
- Ota HC, Smith BG, Alamri A, et al. The IDEAL framework in neurosurgery: a bibliometric analysis. Acta neurochirurgica. 2020;162(12):2939-2947.
- Zarzavadjian Le Bian A, Fuks D, Costi R, et al. Innovation in Surgery: Qualitative Analysis of the Decision-Making Process and Ethical Concerns. Surg Innov. 2018:1553350618789265.
- Webster J. The ten most important biomedical engineering devices. Paper presented at: World Congress on Medical Physics and Biomedical Engineering 20062007.
- Beswick DM, Kaushik A, Beinart D, et al. Biomedical device innovation methodology: applications in biophotonics. Journal of Biomedical Optics. 2017;23(2):021102.
- Stack RS, Harrington RA. Biomedical innovation: A risky business at risk. Science translational medicine. 2011;3(96):96cm23-96cm23.
- Dey M, Ozbolat IT. 3D bioprinting of cells, tissues and organs. In. Vol 10: Nature Publishing Group; 2020:1-3.
- Alvarez P, Sianis A, Brown J, Ali A, Briasoulis A. Chronic disease management in heart failure: focus on telemedicine and remote monitoring. Reviews in Cardiovascular Medicine. 2021;22(2):403-413.
- DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC: Heart failure. 2019;7(11):922-932.
- Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86(5):334-338.
- Gore JC. Artificial intelligence in medical imaging. In. Vol 68: Elsevier; 2020:A1-A4.
- Hashimoto DA, Witkowski E, Gao L, Meireles O, Rosman G. Artificial intelligence in anesthesiology: current techniques, clinical applications, and limitations. Anesthesiology. 2020;132(2):379-394.
- Hessler G, Baringhaus K-H. Artificial intelligence in drug design. Molecules. 2018;23(10):2520.
- Lopez-Jimenez F, Attia Z, Arruda-Olson AM, et al. Artificial intelligence in cardiology: present and future. Paper presented at: Mayo Clinic Proceedings2020.
- Schwartz JG, Kumar UN, Azagury DE, Brinton TJ, Yock PG. Needs-based innovation in cardiovascular medicine: The Stanford Biodesign Process. JACC: Basic to Translational Science. 2016;1(6):541-547.
- Kadakia S, Moore R, Ambur V, Toyoda Y. Current status of the implantable LVAD. General thoracic and cardiovascular surgery. 2016;64(9):501-508.
- Pourdjabbar A, Ang L, Behnamfar O, et al. Robotics in percutaneous cardiovascular interventions. Expert Review of Cardiovascular Therapy. 2017;15(11):825-833.
- Wegermann ZK, Swaminathan RV, Rao SV. Cath Lab Robotics: paradigm change in interventional cardiology? Current Cardiology Reports. 2019;21(10):1-7.
- Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. The Journal of arthroplasty. 2016;31(10):2353-2363.
- Duan X, Wang B, Yang L, Kadakia AR. Applications of 3D printing technology in orthopedic treatment. In. Vol 2021: Hindawi; 2021.
- Campbell KJ, Louie PK, Khorsand DA, Frisch NB, Gerlinger TL, Levine BR. Innovation and entrepreneurship: perspectives from orthopedic surgery. Orthopedics. 2018;41(3):135-140.
- Courvoisier A. The future of academic innovation in the field of medical devices: is innovation still possible in orthopedics? Expert review of medical devices. 2016;13(9):807-813.
- Maher SA, Kyle R, Morrey BF, Yaszemski MJ. Translating orthopaedic technologies into clinical practice: Challenges and Solutions. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2019;27(1):e9-e16.
- Laing R. A History of Neurosurgery. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(2):284-284.
- Nikova A, Birbilis T. The Basic Steps of Evolution of Brain Surgery. Maedica (Bucur). 2017;12(4):297-305.
- Powell M. Recent advances: neurosurgery. BMJ. 1999;318(7175):35-38.
- Potel SR, Marceglia S, Meoni S, Kalia SK, Cury RG, Moro E. Advances in DBS Technology and Novel Applications: Focus on Movement Disorders. Curr Neurol Neurosci Rep. 2022;22(9):577-588.
- Dell'Italia J, Sanguinetti JL, Monti MM, Bystritsky A, Reggente N. Current State of Potential Mechanisms Supporting Low Intensity Focused Ultrasound for Neuromodulation. Front Hum Neurosci. 2022;16:872639.
- Hersh AM, Alomari S, Tyler BM. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int J Mol Sci. 2022;23(8).
- Datta A, Sarmah D, Kaur H, et al. Advancement in CRISPR/Cas9 Technology to Better Understand and Treat Neurological Disorders. Cell Mol Neurobiol. 2022.
- Madsen PJ, Lang SS, Adappa ND, Palmer JN, Storm PB. Pediatric Pituitary Surgery. Otolaryngol Clin North Am. 2022;55(2):477-491.
- Lev-Tov L, Barbosa DAN, Ghanouni P, Halpern CH, Buch VP. Focused ultrasound for functional neurosurgery. J Neurooncol. 2022;156(1):17-22.
- Grailer JG, 3rd, Alhallak K, Antes AL, et al. A Novel Innovation and Entrepreneurship (I&E) Training Program for Biomedical Research Trainees. Acad Med. 2022.
- Arias J, Scott KW, Zaldivar JR, et al. Innovation-Oriented Medical School Curricula: Review of the Literature. Cureus. 2021;13(10):e18498.
- Maloney LM, Hakimi M, Hays T, et al. Learning the Language of Medical Device Innovation: A Longitudinal Interdisciplinary Elective for Medical Students. Acad Med. 2022.
- Niccum BA, Sarker A, Wolf SJ, Trowbridge MJ. Innovation and entrepreneurship programs in US medical education: a landscape review and thematic analysis. Med Educ Online. 2017;22(1):1360722.
- NIHF. Take Your Innovation to the Next Level with Our Collegiate Inventors Competition. 2022; https://www.invent.org/collegiate-inventors Accessed 07/25/2022, 2022.
- CNS. Innovator of the Year. 2022; https://www.cns.org/membership/rpcc-detail/innovator-of-year-2022. Accessed 07/26/2022, 2022.
- Schucht P, Roccaro-Waldmeyer DM, Murek M, et al. Exploring Novel Funding Strategies for Innovative Medical Research: The HORAO Crowdfunding Campaign. J Med Internet Res. 2020;22(11):e19715.
- Servoss J, Chang C, Olson D, Ward KR, Mulholland MW, Cohen MS. The Surgery Innovation and Entrepreneurship Development Program (SIEDP): An Experiential Learning Program for Surgery Faculty to Ideate and Implement Innovations in Health care. J Surg Educ. 2018;75(4):935-941.
- Wong DJ, Miranda-Nieves D, Nandivada P, et al. The Surgical Program in Innovation (SPIN): A Design and Prototyping Curriculum for Surgical Trainees. Acad Med. 2021;96(9):1306-1310.
- Yu Y, Jayasundera KT, Servoss J, et al. A Novel Think Tank Program to Promote Innovation and Strategic Planning in Ophthalmic Surgery. Perioper Care Oper Room Manag. 2021;22.
- Bohl MA, Mooney MA, Sheehy J, et al. The Barrow Innovation Center: A Novel Program in Neurosurgery Resident Education and Medical Device Innovation. Cureus. 2018;10(2):e2142.
- APSA. Funding Opportunities. 2022; https://www.physicianscientists.org/page/FundingOpportunities. Accessed 07/26/2022, 2022.
- AANS. Grants, Fellowships and Awards. 2022; https://www.aans.org/Trainees/Grants-and-Fellowships. Accessed 07/26/2022, 2022.
- CNS. Awards and Resources. 2022; https://www.cns.org/residency/resident-programs/resident-research-grants-awards. Accessed 07/27/2022, 2022.
- AMA. Accelerating Change in Medical Education Innovation Grant Program. 2022. Accessed 07/24/2022, 2022.
- CSNS. Fellowships & Awards. 2022; https://csnsonline.org/fellowships_awards.php. Accessed 08/15/2022, 2022.
- NIH-RTCD. Research Career Development Awards. 2022; https://researchtraining.nih.gov/programs/career-development. Accessed 07/25/2022, 2022.
- NSF. WE INVEST UP TO $2 MILLION IN SEED FUNDING. AND TAKE ZERO EQUITY. 2022; https://seedfund.nsf.gov. Accessed 08/08/22, 2022.
- NSF. NSF's Innovation Corps (I-Corps™). 2022; https://beta.nsf.gov/funding/initiatives/i-corps. Accessed 08/12/2022, 2022.
- NIH-NIBIB. Concept to Clinic: Commercializing Innovation (C3i) Program. 2022; https://www.nibib.nih.gov/research-program/c3i-program. Accessed 08/14/2022, 2022.
- NIH. Research Evaluation and Commercialization Hubs (REACH). 2022; https://seed.nih.gov/programs-for-academics/academic-entrepreneurship-and-product-development-programs/reach. Accessed 08/15/2022, 2022.
- NIH. Other NIH Proof of Concept Programs. 2022; https://seed.nih.gov/programs-for-academics/academic-entrepreneurship-and-product-development-programs/other-nih-proof-of-concept-program Accessed 08/15/2022, 2022.
- Slatnick BL, Truche P, Wu KC, et al. Trends in Surgical Patents Held by Surgeons from 1993 to 2018. Ann Surg. 2021.
- Maisel WH. Medical device regulation: an introduction for the practicing physician. Ann Intern Med. 2004;140(4):296-302.
- Moser P. Innovation Without Patents‐Evidence from the World Fairs. Forthcoming in the. Journal of Law and Economics. 2011.
- Boms O, Shi Z, Mallipeddi N, et al. Integrating innovation as a core objective in medical training. Nature Biotechnology. 2022;40(3):434-437.
- Augustin DA, Yock CA, Wall J, et al. Stanford’s Biodesign Innovation program: Teaching opportunities for value-driven innovation in surgery. Surgery. 2020;167(3):535-539.
- Sharif F, Quinn I. Driving medtech innovation and start-up company formation through successful joint academic/commercial fellowship. BMJ Innovations. 2021;7(2):407-413.
- Makower J. FDA Impact on U.S. Medical Technology Innovation. 2010. http://www.medtecheurope.org/wp-content/uploads/2015/09/01112010_FDA-impact-on-US-medical-technology-innovation_Backgrounder.pdf. Accessed Aug 29, 2022.
- Drlik M. How Much Does it Cost to Develop a Medical Device. 2020. https://starfishmedical.com/assets/StarFish-Whitepaper-Cost-to-Develop-Medical-Devices-July-2020.pdf. Accessed Aug 29, 2022.
- CNS. CNS/CSNS Medical Student Summer Fellowship in Socioeconomic Research. 2022; https://www.cns.org/cns-csns-medical-student-summer-fellowship-in-socioeconomic-research. Accessed 08/17/2022, 2022.
- NIH. Funding for Cancer Training. 2022; https://www.cancer.gov/grants-training/training/funding/k22. Accessed 08/18/2022, 2022.
- NREF. Grant, Fellowship and Award Deadlines. 2022; https://www.nref.org/research/Deadline-Calendar. Accessed 08/18/2022, 222.
- NIH-Seed. Understanding SBIR and STTR. 2022; https://seed.nih.gov/small-business-funding/small-business-program-basics/understanding-sbir-sttr. Accessed 08/15/2022, 2022.
- Gelberman RH, Samson D, Mirza SK, Callaghan JJ, Pellegrini Jr VD. Orthopaedic surgeons and the medical device industry: the threat to scientific integrity and the public trust. JBJS. 2010;92(3):765-777.
- Lexchin J, Fugh-Berman A. A Ray of Sunshine: Transparency in Physician-Industry Relationships Is Not Enough. Journal of General Internal Medicine. 2021;36(10):3194-3198.
- Hopkins B, Yamaguchi JT, Cloney MB, Shlobin NA, Dahdaleh NS. Effects of the physician payments sunshine act on the patient experience and perception of care amongst neurosurgeons: a comparative study of online PRW ratings and industry payments. Clinical Neurology and Neurosurgery. 2019;176:127-132.
- de Lotbiniere-Bassett MP, McDonald PJ. Industry financial relationships in neurosurgery in 2015: analysis of the sunshine act open payments database. World neurosurgery. 2018;114:e920-e925.
- Stumpo V, Staartjes VE, Klukowska AM, et al. Global adoption of robotic technology into neurosurgical practice and research. Neurosurg Rev. 2021;44(5):2675-2687.
- Langer DJ, White TG, Schulder M, Boockvar JA, Labib M, Lawton MT. Advances in intraoperative optics: a brief review of current exoscope platforms. Operative Neurosurgery. 2020;19(1):84-93.
- Montemurro N, Scerrati A, Ricciardi L, Trevisi G. The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. Journal of Clinical Medicine. 2022;11(1):223.
- Ho A, Khan YR, Whitney E, Alastra AJ, Siddiqi J. The Influence of Intraoperative Technology on Neurosurgery Training. Cureus. 2019;11(9):e5769.
- Adapted from Multiple Sclerosis CNS with Callout (Layout). In. https://app.biorender.com/biorender-templates: Biorender.com; 2022.


