Research Article | DOI: https://doi.org/10.58489/2836-8991/001
Unrelated versus related living-donor kidney transplantation is associated with higher acute rejection rate but similar graft survival Running title: Outcomes in living-donor kidney transplantation
- Pedro R. Pereira 1,5,6
- Manuela Almeida 2,5,6
- Bárbara O. Ribeiro 3
- João P. Oliveira 2
- Luísa L. Costa 4
- Jorge Malheiro 2,5,6
- Sofia Pedroso 2,5,6
- La Salete Martins 2,5,6
- Leonídio Dias 2
- Department of Nephrology, Centro Hospitalar de Trás-os-Montes e Alto Douro (CHTMAD) - Vila Real, Portugal
- Department of Nephrology, Centro Hospitalar Universitário do Porto (CHUP) - Porto, Portugal
- Department of Nephrology, Hospital de Braga - Braga, Portugal
- Department of Nephrology, Centro Hospitalar Tondela-Viseu (CHTV) - Viseu, Portugal
- Group of Nephrology, Dialysis and Transplantation, UMIB - Unit for Multidisciplinary Research in Biomedicine, ICBAS – Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, Porto, Portugal.
- ITR - Laboratory for Integrative and Translational Research in Population Health, Porto, Portugal
*Corresponding Author: Pedro R. Pereira
Citation: Pedro R. Pereira, (2022). Unrelated versus related living-donor kidney transplantation is associated with higher acute rejection rate but similar graft survival. Journal of Transplantation Proceedings and Research. 1(1). DOI: 10.58489/2836-8991/001
Copyright: © 2022 Pedro R. Pereira, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 23 August 2022 | Accepted: 20 September 2022 | Published: 20 December 2022
Keywords: living-related donor, living unrelated donor, kidney transplant, graft survival, acute rejection, immunosuppression
Abstract
Kidney transplantation (KT) from living donors has been shown to have multiple benefits compared with that from deceased donors. We sought to compare significant graft outcomes, namely acute rejection (AR), graft function, and survival, between transplant recipients who received a kidney from a living-related donor (LRD) and from a living unrelated donor (LURD). Our cohort comprised 198 donor and recipient pairs undergoing living-donor KT at our center over 10 years. LRD recipients were compared with LURD recipients according to demographic and clinical characteristics, transplant variables (including immunosuppression), graft function, survival, and AR rate. The estimated glomerular filtration rate (eGFR) was similar in both groups over the follow-up time, of 60-65 mL/min (p<0.05 over a 10-year period). Censored graft survival was similar between LRD and LURD recipients (96.9% vs. 98.0% at 5 years and 87.8% vs. 79.4% at 10 years, respectively; p=0.837). LURD recipients had a higher incidence of AR, although LURD recipient status was not an independent risk factor for AR. Multivariate analysis showed that human leukocyte antigen (HLA) -DR mismatch (MM) was an independent predictor of AR (hazard ratio [HR] 2.256, p<0.05). HLA-A and HLA-B MM did not affect the AR HR between the groups. Graft function and censored-death survival rates were similar between the LURD and LRD KT recipients. HLA-DR MM was an independent risk factor for AR.
Introduction
Kidney transplantation (KT) has shown to improve survival and long-term outcomes in patients with end-stage kidney failure [1]. Over the last decades, the number of patients on KT waitlist has been steadily increasing [2]. In the setting of organ scarcity, living KT allows to increase donor pool and to reduce waiting time for KT [3].
Living donors can be classified as living-related donors (LRD) or as living-unrelated donors (LURD). LRD are defined as being genetically related to the transplant recipient, such as parents, siblings, or children. LURD, on the other hand, are not genetically related to the transplant recipient: they could be someone with whom the recipient has an emotional connection, such as a spouse or a friend, as well as an unacquainted person, such as an altruistic donor or a donor from a kidney paired exchange (KPE) program. With policies and legislative issues varying between countries, some countries do not allow LURD KT or KPE programs. In Portugal, legislation allowing genetically unrelated transplantation was passed in 2007, and is based on evidence showing that transplants from unrelated living donors too have better outcomes compared with transplants from deceased donors [4].
Several studies have focused on comparing the outcomes between LRD and LURD transplant recipients. Most studies have shown similar graft survival between recipients of these two types of living donation [5-11], while some studies have shown a better survival of LRD recipients compared to LURD recipients [12,13]. A recent study with 14 370 patients reported similar patient and overall graft survival in LRD and LURD recipients, while a higher death censored graft failure in LURD recipients was noticeable [14]. Moreover, some studies have reported higher rates of vascular rejection in LURD recipients [10,15], while others observed similar rates of acute rejection (AR) between both types of living KT [8]. Incidence of chronic allograph nephropathy has also been shown not to be different between LRD and LURD recipients, as well as rates of other post-transplant complications [15].
In this study we aimed to compare graft function and survival, as well as rates of AR in transplant recipients from LRD and LURD, evaluating the first ten years of our center’s experience after the introduction of LURD KT in Portugal.
Materials And Methods
Study population
We retrospectively reviewed the clinical data of adult donor and recipient pairs undergoing living donor KT (LDKT) at our institution between January 2008 and December 2017 (n = 210). After exclusion of 7 recipients who had been submitted to ABO-incompatible KT, and of 5 (2.5%) patients who had primary loss of KT, the remaining 198 recipients defined our study cohort. Regarding these primary non-function cases, four (1.9%) were LRD recipients and one (0.5%) was a LURD recipient (p=0.650).
Baseline data and graft outcomes
Baseline demographic, anthropomorphic, analytical, and clinical data were collected from both recipients and donors. Transplant data were also analyzed. Human leukocyte antigen (HLA) -incompatible KT refers to cases in which transplants are performed in the presence of preformed donor-specific antibodies (DSA). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to predict the estimated glomerular filtration rate (eGFR). Delayed graft function (DGF) was defined as the need for dialysis in the first week after transplantation. Graft biopsies were performed for indication. Each recipient was followed up until the end of June 2019, the date of death, graft loss, or loss during follow-up. The study protocol was reviewed and approved by the institutional ethical review and hospital administration boards in accordance with the recommendations of the Declaration of Helsinki and European Data Protection Regulations.
Immunosuppression (IS) and desensitization protocols
Induction therapy was used in most patients, with an anti-IL-2 receptor monoclonal antibody (Basiliximab Novartis", 20 mg twice on days 0 and 4) or polyclonal anti-thymocyte globulin (ATG Fresenius", 3 mg/kg for 5–7 days). ATG was primarily used in HLA-incompatible KT and retransplants. All enrolled recipients had similar triple maintenance immunosuppression consisting of oral tacrolimus, mycophenolate mofetil (MMF), and methylprednisolone (MP)/prednisolone. Further details of our regimen have already been published [16].
HLA-incompatible KT received desensitization with intravenous immunoglobulin (2 g/kg) at transplant (0.5 g/kg immediately before transplant, and at days 1, 2, and 3) and 1 month after transplantation (1 g/kg in two consecutive days), and a dose of rituximab (375 mg/ m2) on day 3 post-transplant. Given the strength of preformed anti-HLA DSA and flow cytometry crossmatch results, six patients also underwent plasmapheresis every other day (first session 3 days before transplant, for a total of 6–9 sessions) [16].
Statistical analysis
Continuous data are described as mean ± standard deviation (SD) or median (interquartile range [IQR]), and categorical data are expressed as numbers and percentages. Categorical data were compared using Pearson’s chi-square test or Fisher’s exact test, and continuous variables were compared using Student’s t-test or Mann–Whitney U-test, as appropriate.
AR and graft survival curves were visualized using the Kaplan–Meier method. Comparisons between patient groups were performed using the log-rank test. In cases of death with a functioning graft, the time was censored at the time of death. Potential predictors of AR and graft failure were explored using univariate and multivariate Cox proportional hazard models. In all multivariable models, independent predictors were identified using a backward elimination method, with a P-value < 0>
A 2-sided P-value of < 0>
Results:
Patients’ characteristics
Our study cohort comprised 198 recipients; 59% (n=116) had LRD KT, and 41% (n=82) had LURD KT. The main group characteristics of living donor pairs and transplants based on LRD and LURD are shown in Table 1.
Mean recipient age at the time of KT was lower in LRD comparing to LURD recipients (35.9±12.2 vs. 48.5±10.9 years old, p< 0 xss=removed>. The percentage of preemptive KT was 28% (n=33) in the LRD group and 21% (n=17) in the LURD group (p=0.061). HLA-A mismatches (MM) were significantly higher in LURD recipients, of 1.40±0.61, compared to 0.67±0.59 in LRD recipients. HLA-B MM was also higher in LURD recipients, of 1.66±0.50 compared to 0.80±0.64 in LRD recipients, as well as HLA-DR MM, which were 0.67±0.59 for LURD recipients and 0.67±0.59 for LRD recipients (P<0>
The immunosuppression induction regimen included basiliximab in 86% (n=100) of LRD recipients and 88% (n=72) of LURD recipients, ATG in 10% (n=12) of LRD recipients and 11% (n=9) of LURD recipients; 3% (n=4) of LRD recipients, and 1% (n=1) of LURD recipients had no induction immunosuppression (p=0.614). The maintenance immunosuppression regimen included triple immunosuppression with tacrolimus, mofetil mycophenolate, and prednisone in 97% (n=113) of the LRD recipients and 98% (n=79) of the LURD recipients. Median follow-up was of 5.0 years [IQR:3.3 – 7.2].
Acute rejection
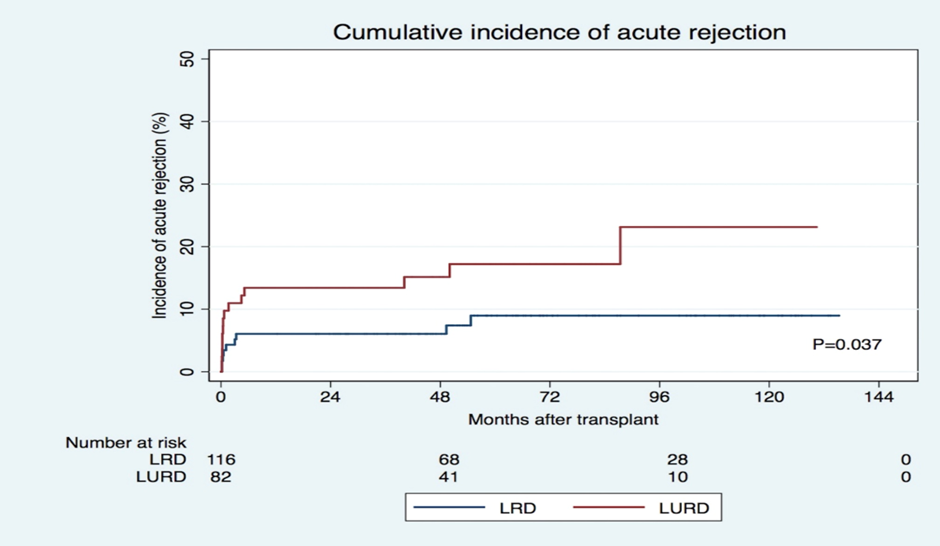
AR was observed in 23 recipients (12%), of which 13 had acute cellular rejection (ACR) and 10 had antibody-mediated rejection (ABMR) (Table 2). Nine cases (8%) of AR were identified in LRD recipients, while LURD recipients had 14 cases (17%) of AR (p=0.044). The cumulative incidence of AR during the follow-up period is shown in Figure 1. The days until AR and the incidence of ABMR and T cell-mediated (cellular) rejection (TCMR) were similar in both groups.
In the univariate analysis, LURD recipients were at an increased risk of AR (HR= 2.348; p=0.046). However, multivariate analysis showed that LURD was not an independent risk factor for AR (after adjustment for recipient and donor age, sex, immunosuppression induction regimen, previous time on KRT, type of previous KRT, re-transplantation rate, donor eGFR, prevalence of HLA-A MM, HLA-B-MM, and receptor BMI) (Table 3). In contrast, HLA-DR MM increased the HR of AR in both groups (HR 2.256, p=0.011). HLA-A and HLA-B MM did not affect the AR HR between the groups of patients. Additionally, KT occurring in the 2008–2012-time frame was associated with a significantly higher risk of AR (HR 2.480, p=0.039).
When ACR and ABMR were analyzed separately, higher BMI was associated with a higher risk of ACR (HR 1.179, p=0.013) (Table 4), and HLA-DR MM had an independent impact on ABMR (HR 2.892, p=0.045) and HLA incompatibility (HR 5.070, p=0.012) (Table 5). LURD KT was not significantly associated with any of the rejection types in either univariate or multivariate analysis.
Graft and patient survival
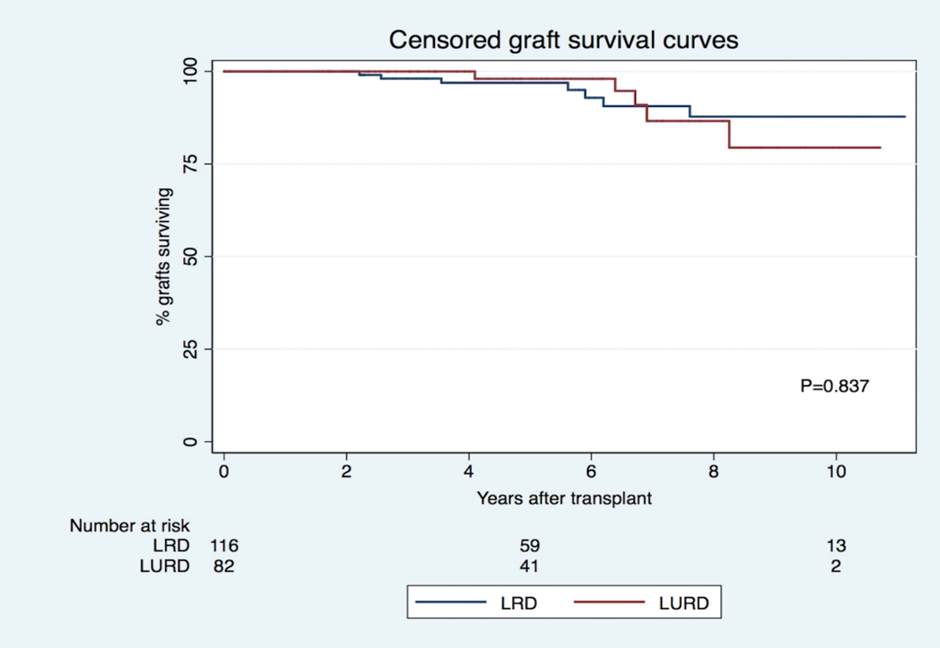
Censored graft survival was similar for LRD and LURD recipients (96.9% vs. 98.0% at 5 years and 87.8% vs. 79.4% at 10 years, p=0.837, respectively) (Figure 2), which remained true after adjustment for several factors. Recipient age (HR 0.938, p<0>16.576, p< 0 xss=removed>and presence of preformed DSA (HR 3.387, p<0>, 99%/94% for LRD recipients with no AR, 78%/47% for LURD recipients with AR, 100%/100% for LURD recipients with no AR, and 91% / 28% for LURD recipients and AR (overall p<0 xss=removed>Patient survival was similar in both groups (1 (1%) death in the LRD group and 1 (1%) death in the LURD group (p=0.422]).
Graft function
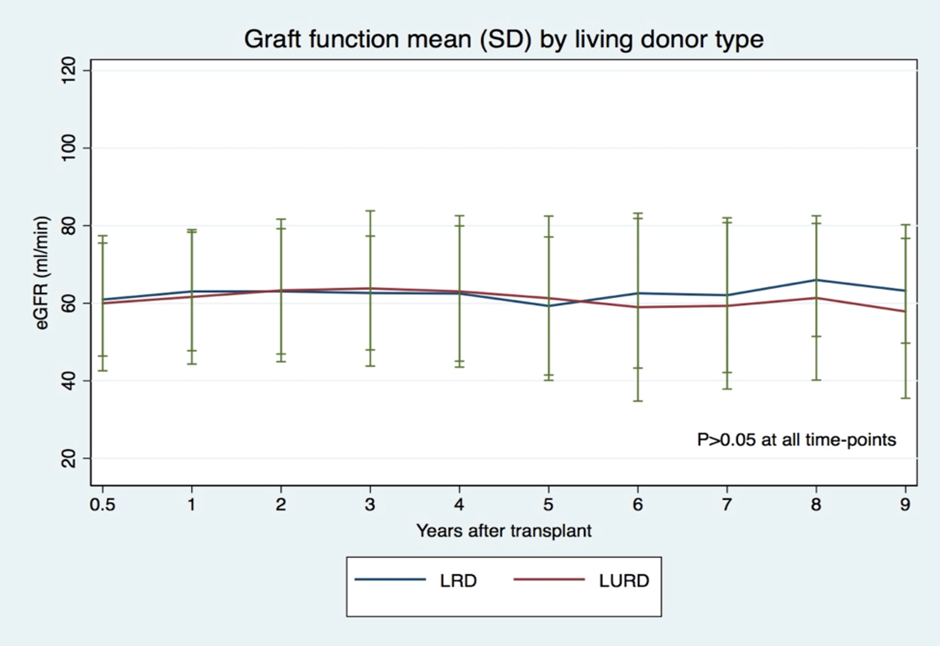
Graft function was similar in both groups over the follow-up period: around 60-65 mL/min (p>0.05, in all evaluated time-points) (Figure 3).
Discussion
Living KT can mitigate organ scarcity and help reduce KT waitlists(3). Evaluating the impact of living donor sources on long-term outcomes may potentially allow the optimization of donor matching and immunosuppression to improve results(17). In this study, we aimed to compare graft function and survival between LURD and LRD transplant recipients. Additionally, we studied AR occurrence in both groups.
In our study, graft function was similar in both groups during the medium and long-term follow-up periods. Other studies have found that the donor source does not significantly influence graft function in living donation(10, 14). Censored graft survival was also comparable between LURD and LRD patients, which is in line with most previous studies suggesting graft survival to be similar for both groups of patients(7). In a recent large-scale study, however, LURD had higher death censored graft failure than LRD recipients(14), which contrasts with our results; although the authors stated that they could not explain this finding, they speculated that it could be due to old age and a high proportion of patients with type II diabetes mellitus and hypertension as primary kidney disease among LURD transplant recipients. Long-term follow-up and large-scale studies are necessary to confirm these results.
Patient survival was also similar between both groups, with 1 (1%) death in the LRD group and 2 (2%) deaths in the LURD group. A United States study from 1998(12) showed that 10-year patient survival among recipients of LURD transplants was worse than that of LRD transplants (86% vs. 63%, respectively), although these findings have not been replicated in other more recent studies, which have consistently shown similar survival rates between LRD and LURD KT recipients(10, 14).
In our study, the univariate analysis showed that LURD recipients had a higher incidence of AR. However, in the multivariate analysis, LURD recipient status was not found to be an independent risk factor for AR, consistent with findings from previous studies(8). However, the presence of HLA-DR MM predicted an increased risk of AR, regardless of the donor origin. LURD recipients had higher HLA-DR MM, which explains why, despite having a higher rate of AR in multivariate analysis, LURD recipient status did not predict AR, while HLA-DR MM did (Tables 1 and 3). In contrast, HLA-A and HLA-B MM did not affect the AR between the groups. HLA-MM is recognized as a strong risk factor for the development of AR(18, 19) and HLA-DR MM in particular has been shown to strongly influence KT outcomes(20), namely AR(21, 22). More recently, HLA-DR epitope mismatch has been shown to be an independent predictor of ABMR(23). A recent study using random forest analysis in the UNOS database identified HLA-DR as an important variable for acute rejection among black kidney transplant recipients in the United States(24). In pancreatic transplantation, HLA-DR MM has been shown to independently predict acute rejection(25) – an effect that might be reproducible in KT. The days until AR were similar in LURD and LUD recipients. Other adverse outcomes are associated with HLA-MM. In the case of deceased donors, HLA matching has been shown to correlate with renal allograft and patient survival, even in the absence of preformed DSA(26, 27), but few studies have evaluated this relationship in living donor transplants. In one study with first adult transplants from deceased donors in the United States between 1987 and 2013, a significant linear relationship between HLA MM and graft survival was identified, with 1 MM conferring a 13% higher risk and 6 MM conferring a 64% higher risk of allograft failure(26). In another study, 83 0-HLA MM patients were matched to 407 controls with more than 0-HLA MM, with the authors reporting no differences in death-censored graft survival or patient survival for both groups(28). Our data reinforce the importance of HLA-DR matching and its association with graft survival and incidence of rejection(25, 29, 30).
The hurdle associated with high HLA MM in LDKT may potentially be managed by the introduction of compatible pairs in a KPE program, which has been possible in Portugal since legislation concerning the National KPE program was amended in 2021. Careful immunological risk profiling, including improved HLA and epitope analysis, could also improve these results. In addition, a clear definition of the inclusion criteria for compatible pairs in KPE is crucial. A recent report that reviewed the first 9 years of KPE transplants from the National Kidney Registry in the United States showed a 27% lower 5-year graft failure rate compared to traditional direct living donor transplants(31), and improved transplant outcomes have been attributed to improved antibody avoidance. In the setting of an increasingly hypersensitized population of KT candidates worldwide, surely the optimal choice type of KT is a low HLA mismatch transplant.
In our population, the preemptive KT rate was similar in both groups, 28% (n=33) for LRD recipients and 21% (n=12) for LURD recipients. As waiting time on dialysis is considered the strongest modifiable risk factor for KT outcomes(32), increasing this rate would certainly improve our results.
The major limitation of this study is the sample size. However, it should be emphasized that our data refers to a single-center population, with similar background demographic and clinical features, submitted to KT by the same multidisciplinary team, which delivered the same standards of patient care. This allowed the retrieval of robust data for statistical analysis; thus, valid conclusions can still be ascertained. In addition, the fact that this was a retrospective study rather than a prospective study designed to assess KT outcomes with formal event adjudication implies that the level of evidence is not as high as would be derived from a clinical trial. On the other hand, our results are significant, as our center is currently responsible for more than half of all LDKT performed in Portugal.
Conclusion
In conclusion, graft function and censored-death survival rates were similar between LURD and LRD KT recipients in our study. AR was higher in LURD recipients, although the LURD recipient status was not an independent risk factor for AR. HLA-DR MM was an independent predictor of AR, while HLA-A and HLA-B MM did not affect AR HR between groups of patients.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia – FCT the Unit for Multidisciplinary Research in Biomedicine (UMIB) (UIDB/00215/2020, UIDP/00215/2020 and LA/P/0064/2020).
Figures
Tables:
Table 1. Baseline characteristics of living donor pairs and transplants, based on LRD and LURD.
| Total N=198 | LRD N=116 (59%) | LURD N=82 (41%) | P |
Recipient |
|
|
|
|
Age R, mean ±SD | 41.1±13.2 | 35.9±12.2 | 48.5±10.9 | <0> |
Sex R F, n (%) | 56 (28) | 36 (31) | 20 (24) | 0.307 |
BMI R, mean±SD | 23.9±3.9 | 23.2±3.9 | 24.9±3.9 | 0.004 |
Time on dialysis before KT (months), median (IQR) | 13.9 (0-30.3) | 12.6 (0-27.2) | 16.3 (3.9-32.3) | 0.125 |
RRT pre-KT, n (%) Preemptive HD PD |
50 (25) 106 (54) 42 (21) |
33 (28) 54 (47) 29 (25) |
17 (21) 52 (63) 13 (16) | 0.061
|
Donor |
|
|
|
|
Age D, mean ± SD | 48.1±10.5 | 47.4±11.4 | 49.1±9.2 | 0.265 |
Sex D F, n (%) | 143 (72) | 80 (69) | 63 (77) | 0.224 |
BMI D, mean±SD | 25.3±3.5 | 25.2±3.5 | 25.4±3.5 | 0.798 |
Predonation eGFR, mean ± SD | 100.2±14.3 | 101.4±14.0 | 98.4±14.6 | 0.144 |
Left kidney donated, n (%) Missing: 9 | 156 (83) | 89 (81) | 67 (85) | 0.486 |
Transplant |
|
|
|
|
Year of KT, n (%) 2008-2012 2013-2017 |
71 (36) 127 (64) |
42 (36) 74 (64) |
29 (35) 53 (65) | 0.903
|
Retransplant, n (%) | 27 (14) | 18 (16) | 9 (11) | 0.359 |
Calculated PRA >0%, n (%) | 60 (30) | 37 (32) | 23 (28) | 0.562 |
HLA-incompatible KT, n (%) | 22 (11) | 14 (12) | 8 (10) | 0.610 |
HLA-A MM, mean±SD | 0.94±0.68 | 0.63±0.52 | 1.39±0.62 | <0> |
HLA-A MM, n (%) 0 1 2 |
51 (26) 107 (54) 40 (20) |
45 (39) 69 (59) 2 (2) |
6 (7) 38 (46) 38 (46) | <0>
|
HLA-B MM, mean±SD | 1.16±0.72 | 0.80±0.64 | 1.66±0.50 | <0> |
HLA-B MM, n (%) 0 1 2 |
38 (19) 91 (46) 69 (35) |
37 (32) 65 (56) 14 (12) |
1 (1) 26 (32) 55 (67) | <0>
|
HLA-DR MM, mean±SD | 0.97±0.69 | 0.67±0.59 | 1.40±0.61 | <0> |
HLA-DR MM, n (%) 0 1 2 |
50 (25) 103 (52) 45 (23) |
45 (39) 64 (55) 7 (6) |
5 (6) 39 (48) 38 (46) | <0>
|
IS Induction, n (%) Without Basiliximab ATG |
5 (3) 172 (87) 21 (11) |
4 (3) 100 (86) 12 (10) |
1 (1) 72 (88) 9 (11) | 0.614
|
Maintenance IS, n (%) TAC + MMF+pred others |
192 (97) 6 (3) |
112 (97) 4 (3) |
80 (98) 2 (2) | 1
|
DGF, n (%) | 8 (4) | 5 (4) | 3 (4) | 1 |
Follow-up (years), median (IQR) | 5.1 (3.3-7.2) | 5.2 (3.4-8.3) | 4.9 (3.0-6.9) | 0.327 |
Abbreviations: LRD: living related donor; LURD: living unrelated donor; R: recipient; SD: standard deviation; F: female; BMI: body mass index; KT: kidney transplant; IQR: interquartile range; RRT: renal replacement therapy; HD: hemodialysis; PD: peritoneal dialysis; D: donor; eGFR: estimated glomerular filtration rate; PRA: panel-reactive antibodies; HLA: human leukocyte antigen; MM: mismatch; ATG: antithymocyte globulin; TAC: tacrolimus; MMF: mofetil mycophenolate; pred: prednisone; DGF: delayed graft function.
Table 2. Impact of LURD vs LRD transplants in acute rejection.
| Total N=198 | LRD N=116 (59%) | LURD N=82 (41%) | P |
Acute rejection (AR) n (%) | 23 (12) | 9 (8) | 14 (17) | 0.044 |
Days to AR, median (IQR) | 21 (9-154) | 34 (12-100) | 18 (9-154) | 0.900 |
ACR (%) | 13 (7) | 5 (4) | 8 (10) | 0.128 |
Days to ACR, median (IQR) | 16 (12-91) | 34 (16-91) | 14 (7-92) | 0.305 |
Antibody-mediated rejection (ABMR), n (%) | 10 (5) | 4 (3) | 6 (7) | 0.324 |
Days to ABMR, median (IQR) | 61 (9-1480) | 53 (6-790) | 613 (9-1502) | 0.284 |
Abbreviations: LRD: living related donor; LURD: living unrelated donor; AR: acute rejection; ACR: acute cellular rejection; ABMR: antibody mediated rejection; IQR interquartile range.
Table 3. Predictors of acute rejection.
| HR (CI 95%) | P |
Univariate analysis |
|
|
LURD | 2.348 (1.016-5.427) | 0.046 |
Multivariate analysis 1* |
|
|
HLA-DR MM | 2.256 (1.205-4.223) | 0.011 |
Year of KT, 2008-2012 | 2.480 (1.047-5.874) | 0.039 |
*Adjusted to R age, D age, sex, induction IS, IS of maintenance, retransplant rate, months on RRT, type of dialysis/preemptive, D eGFR, HLA A MM, HLA B MM, KT HLAi, and R BMI.
Abbreviations: HR: hazard ration; LURD: living unrelated donor; HLA: human leukocyte antigen; MM: mismatch; KT: kidney transplant.
Table 4. Predictors of acute cellular rejection (ACR).
| HR (CI 95%) | P |
Univariate analysis |
|
|
LURD | 2.345 (0.767-7.169) | 0.135 |
Multivariate analysis* |
|
|
BMI R | 1.179 (1.035-1.344) | 0.013 |
* Adjusted to R age, D age, R sex, D sex, induction IS, IS of maintenance, retransplant rate, months on RRT, type of dialysis/preemptive, D eGFR, HLA A MM, HLA B MM, KT HLAi, and KT time period.
Abbreviations: HR: hazard ration; LURD: living unrelated donor; BMI: body mass index.
Table 5. Predictors of antibody mediated rejection (ABMR).
| HR (CI 95%) | P |
Univariate analysis |
|
|
LURD | 2.253 (0.634-8.006) | 0.209 |
Multivariate analysis* |
|
|
HLA-DR MM | 2.892 (1.024-8.167) | 0.045 |
HLAi | 5.070 (1.422-18.070) | 0.012 |
* Adjusted to R age, D age, R sex, D sex, induction IS, IS of maintenance, retransplant rate, months on RRT, type of dialysis/preemptive, D eGFR, HLA A MM, HLA B MM, R BMI, and KT time period.
Abbreviations: HR: hazard ration; LURD: living unrelated donor; HLA: human leukocyte antigen; MM: mismatch; HLAi: incompatible HLA transplantation.
Table 6. Predictors of censored graft failure.
| HR (IC 95%) | P |
Univariate analysis |
|
|
LURD | 1.128 (0.357-3.572) | 0.837 |
Multivariate analysis* |
|
|
Acute rejetion | 16.576 (4.444-61.822) | <0> |
HLAi | 3.387 (1.010-11.360) | 0.048 |
*Adjusted to R age, D age, R sex, D sex, induction IS, IS of maintenance, retransplant rate, months on RRT, type of dialysis/preemptive, D eGFR, HLA A MM, HLA B MM, HLA DR MM, R BMI, and KT time period.
Abbreviations: HR: hazard ration; LURD: living unrelated donor; HLAi: incompatible HLA transplantation.
Abbreviations:
ABMR, antibody-mediated rejection
ACR, acute cellular rejection
AR, acute rejection
ATG, anti-thymocyte globulin
BMI, body mass index
DGF, delayed graft function
DSA, donor specific antibody
eGFR, estimated glomerular filtration rate
HLA, human leucocyte antigen
HR, hazard ratio
KPE, kidney paired exchange
KT, kidney transplantation
LD, living donor
LDKT, living donor kidney transplantation
LRD, living related donor
LURD, living unrelated donor
MM, mismatch
HR, hazard ration
RRT, renal replacement therapy
References
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. (1999), Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med.;341(23):1725-30.
View at Publisher | View at Google Scholar - Clayton LM, Rizzolo D, Nair V. (2018), Kidney transplant wait list: Review and current trends. Jaapa; 31(10):1-5.
View at Publisher | View at Google Scholar - Hou S. (2000), Expanding the kidney donor pool: ethical and medical considerations. Kidney Int; 58(4):1820-36.
View at Publisher | View at Google Scholar - RepúblicaAd. Lei nº22/2007 de 29. de Junho: Colheita e transplante de órgãos, tecidos e células de origem humana. Diário da República; 29 de junho de 2007.
View at Publisher | View at Google Scholar - Taylor GS, Prather JC, Norman DJ, de Mattos AM, Mogilishetty G, Conlin MJ, et al. (2005), Living unrelated donor renal transplantation: a single center experience. J Urol; 174(1):223-5.
View at Publisher | View at Google Scholar - Simforoosh N, Basiri A, Fattahi MR, Einollahi B, Firouzan A, Pour-Reza-Gholi F, et al. (2006), Living unrelated versus living related kidney transplantation: 20 years' experience with 2155 cases. Transplant Proc; 38(2):422-5.
View at Publisher | View at Google Scholar - Simforoosh N, Basiri A, Tabibi A, Javanmard B, Kashi AH, Soltani MH, et al. (2016), Living Unrelated Versus Related Kidney Transplantation: A 25-Year Experience with 3716 Cases. Urol J;13(1):2546-51.
View at Publisher | View at Google Scholar - Ahmad N, Ahmed K, Khan MS, Calder F, Mamode N, Taylor J, et al. (2008), Living-unrelated donor renal transplantation: an alternative to living-related donor transplantation? Ann R Coll Surg Engl; 90(3):247-50.
View at Publisher | View at Google Scholar - Kizilisik AT, Ray JM, Nylander WA, Langone AJ, Helderman JH, Shaffer D. (2004), Living donor kidney transplantation in a Veterans Administration medical center. Am J Surg; 188(5):611-3.
View at Publisher | View at Google Scholar - Matter YE, Nagib AM, Lotfy OE, Alsayed AM, Donia AF, Refaie AF, et al. (2016), Impact of Donor Source on the Outcome of Live Donor Kidney Transplantation: A Single Center Experience. Nephrourol Mon; 8(3): e34770.
View at Publisher | View at Google Scholar - Santori G, Barocci S, Fontana I, Bertocchi M, Tagliamacco A, Biticchi R, et al. (2012), Kidney transplantation from living donors genetically related or unrelated to the recipients: a single-center analysis. Transplant Proc; 44(7):1892-6.
View at Publisher | View at Google Scholar - D'Alessandro AM, Pirsch JD, Knechtle SJ, Odorico JS, Van der Werf WJ, Collins BH, et al. (1998), Living unrelated renal donation: the University of Wisconsin experience. Surgery;124(4):604-10; discussion 10-1.
View at Publisher | View at Google Scholar - Bellini MI, Nozdrin M, Pengel L, Knight S, Papalois V. (2022), How good is a living donor? Systematic review and meta-analysis of the effect of donor demographics on post kidney transplant outcomes. J Nephrol.
View at Publisher | View at Google Scholar - Abd ElHafeez S, Noordzij M, Kramer A, Bell S, Savoye E, Abad Diez JM, et al. (2021), The association of living donor source with patient and graft survival among kidney transplant recipients in the ERA-EDTA Registry - a retrospective study. Transpl Int; 34(1):76-86.
View at Publisher | View at Google Scholar - Gheith O, Sabry A, El-Baset SA, Hassan N, Sheashaa H, Bahgat S, et al. (2008), Study of the effect of donor source on graft and patient survival in pediatric renal transplant recipients. Pediatr Nephrol; 23(11):2075-9.
View at Publisher | View at Google Scholar - Silva F, Malheiro J, Pestana N, Ribeiro C, Nunes-Carneiro D, Mandanelo M, et al. (2020), Lower donated kidney volume is associated with increased risk of lower graft function and acute rejection at 1 year after living donor kidney-a retrospective study. Transpl Int; 33(12):1711-22.
View at Publisher | View at Google Scholar - Montgomery RA, Tatapudi VS, Leffell MS, Zachary AA. (2018), HLA in transplantation. Nat Rev Nephrol; 14(9):558-70.
View at Publisher | View at Google Scholar - Mota A, Figueiredo A, Cunha MF, Bastos M, Pratas J, Furtado L. (2003), Risk factors for acute rejection in 806 cyclosporine-treated renal transplants: a multivariate analysis. Transplant Proc; 35(3):1061-3.
View at Publisher | View at Google Scholar - KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009);9 Suppl 3: S1-155.
View at Publisher | View at Google Scholar - Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, et al. (2013), Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant; 13(12):3114-22.
View at Publisher | View at Google Scholar - McKenna RM, Lee KR, Gough JC, Jeffery JR, Grimm PC, Rush DN, et al. (1998), Matching for private or public HLA epitopes reduces acute rejection episodes and improves two-year renal allograft function. Transplantation; 66(1):38-43.
View at Publisher | View at Google Scholar - Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. (2015), The Synergistic Effect of Class II HLA Epitope-Mismatch and Nonadherence on Acute Rejection and Graft Survival. Am J Transplant; 15(8):2197-202.
View at Publisher | View at Google Scholar - Tafulo S, Malheiro J, Santos S, Dias L, Almeida M, Martins S, et al. (2019), Degree of HLA class II eplet mismatch load improves prediction of antibody-mediated rejection in living donor kidney transplantation. Hum Immunol; 80(12):966-75.
View at Publisher | View at Google Scholar - Thongprayoon C, Jadlowiec CC, Leeaphorn N, Bruminhent J, Acharya PC, Acharya C, et al. (2021), Feature Importance of Acute Rejection among Black Kidney Transplant Recipients by Utilizing Random Forest Analysis: An Analysis of the UNOS Database. Medicines (Basel); 8(11).
View at Publisher | View at Google Scholar - Rudolph EN, Dunn TB, Mauer D, Noreen H, Sutherland DE, Kandaswamy R, et al. (2016), HLA-A, -B, -C, -DR, and -DQ Matching in Pancreas Transplantation: Effect on Graft Rejection and Survival. Am J Transplant; 16(8):2401-12.
View at Publisher | View at Google Scholar - Williams RC, Opelz G, McGarvey CJ, Weil EJ, Chakkera HA. (2016), The Risk of Transplant Failure with HLA Mismatch in First Adult Kidney Allografts from Deceased Donors. Transplantation; 100(5):1094-102.
View at Publisher | View at Google Scholar - Zachary AA, Leffell MS. (2016), HLA Mismatching Strategies for Solid Organ Transplantation - A Balancing Act. Front Immunol; 7:575.
View at Publisher | View at Google Scholar - Casey MJ, Wen X, Rehman S, Santos AH, Andreoni KA. (2015), Rethinking the advantage of zero-HLA mismatches in unrelated living donor kidney transplantation: implications on kidney paired donation. Transpl Int; 28(4):401-9.
View at Publisher | View at Google Scholar - Doxiadis, II, de Fijter JW, Mallat MJ, Haasnoot GW, Ringers J, Persijn GG, et al. (2007), Simpler and equitable allocation of kidneys from postmortem donors primarily based on full HLA-DR compatibility. Transplantation; 83(9):1207-13.
View at Publisher | View at Google Scholar - Halleck F, Khadzhynov D, Liefeldt L, Schrezenmeier E, Lehner L, Duerr M, et al. (2016), Immunologic outcome in elderly kidney transplant recipients: is it time for HLA-DR matching? Nephrol Dial Transplant; 31(12):2143-9.
View at Publisher | View at Google Scholar - Flechner SM, Thomas AG, Ronin M, Veale JL, Leeser DB, Kapur S, et al. (2018), The first 9 years of kidney paired donation through the National Kidney Registry: Characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant; 18(11):2730-8.
View at Publisher | View at Google Scholar - Meier-Kriesche HU, Kaplan B. (2002), Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation; 74(10):1377-81.
View at Publisher | View at Google Scholar