Research Article | DOI: https://doi.org/10.58489/2836-2217/014
Synthesis And Antibacterial Activity of 6-Bromo-2-(O-Aminophenyl)-3-Amino-Quinazolin-4(3h)-One From 6-Bromo,2-(O-Aminophenyl)-3,1-Benzoxazin-4(3h)-One
- Osarumwense Peter Osarodion 1
- Department of Chemical Science, Ondo State University of Science and Technology, Okitipupa, Ondo State, Nigeria.
*Corresponding Author: Osarumwense Peter Osarodion
Citation: Osarumwense Peter Osarodion, (2023). Synthesis And Antibacterial Activity of 6-Bromo-2- (O-Aminophenyl) -3- Amino-Quinazolin- 4(3h) - One From 6-Bromo, 2 - (O-Aminophenyl) -3, 1-Benzoxazin-4(3h) - One. Journal of Clinical Case Reports and Trails. 1(3). DOI: 10.58489/2836-2217/014
Copyright: © 2023 Osarumwense Peter Osarodion, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 05 April 2023 | Accepted: 13 April 2023 | Published: 19 April 2023
Keywords: Antibacterial activity; Quinazolinone derivatives; 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one; 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one.
Abstract
Introduction: Quinazolinone derivatives reveal various medicinal properties such as analgesic, anti-inflammatory and anticancer activities, as well as antimicrobial activity. These heterocycles are valuable intermediates in organic synthesis.Methods/Experimental: The compound, 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one (1) was synthesized by dissolving 5-bromo anthranillic acid in 100 ml of pyridine. To this reaction mixture o-amino benzoyl chloride stirring at room temperature for 30 minutes this was refluxed with 75 mL of hydrazine hydrate for 3 hrs at 120-1300C. the reaction mixture was allowed to cool to room temperature to give 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one (2). These Compounds were evaluated for their bacterialrial activity (against some gram positive and gram negative microorganism) and antifungal activity (against Candida albicans). Study Design: This study was experimentally design and the antibacterial activity was evaluated against some microorganism, Staphylococcus aureus, Bacillus species, Aspergillus Species, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, and candida albicans Result: The compounds exhibited significant antibacterial activity with a zone of inhibition in the range of 10 – 16mm in comparison to control. Conclusions: From our findings, the compounds synthesized have higher antibacterial activities as compared to Ciprofloxicin (CPX) and Ketonaxol (PEF) standard antibacterial drugs.
Introduction
Quinazoline derivatives, which belong to the N-containing heterocyclic compounds, have caused universal concerns due to their widely and distinct biopharmaceutical activities. Researchers have already determined many therapeutic activities of quinazoline derivatives, including anti-cancer [1–4], anti-inflammation [5, 6], anti-bacterial [7–10], analgesia [5, 9], anti-virus [11], anti-cytotoxin [12], anti-spasm [9, 13], anti-tuberculosis [14], anti-oxidation [15], anti-malarial [16], anti-hypertension [17], anti-obesity [18], anti-psychotic [19], anti-diabetes [20], etc.
Quinazolinone derivatives have been attracting growing attention from medicinal and agricultural chemists, owing to their diverse biological activities, such as antibacterial [21–27], antifungal [28–30], antiviral [31,32], antitumor [33,34] and anticonvulsant [35] activities.
Heterocyclic chemistry comprises at least half of all organic chemistry research worldwide. In particular, heterocyclic structures form the basis of many pharmaceutical, agrochemical and veterinary products. Among a wide variety of nitrogen heterocycles that have been explored for developing role in medicinal chemistry and subsequently have emerged as a pharmacophore [36].
This research was aimed at synthesis of 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one and 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one and investigating them for their antibacterial activity and to obtain more precise information about the course of reaction.
Chemistry
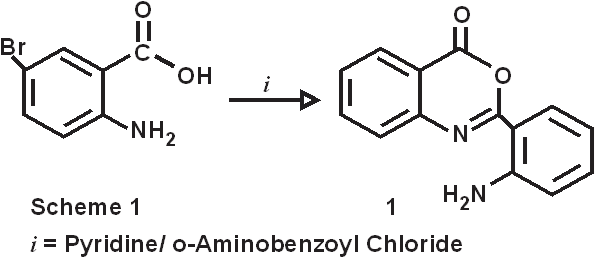
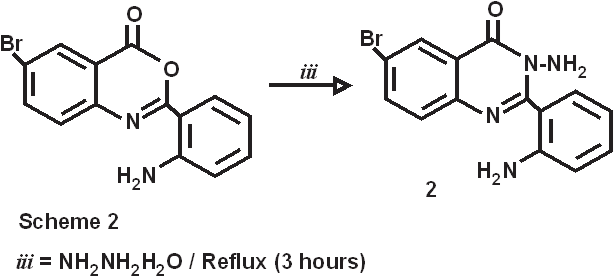
The introduction of 2-amino substituent is a successful strategy to improve the chemical stability of benzoxazinone. Due to the antibacterial and pharmacological activities of 4(3H)-quinazolinone derivatives, 2,3-disubstituted derivative of quinazoline-4-one were synthesized via the interaction of the benzoxazinone derivative with nitrogen nucleophile with the aim of obtaining more pricise information about the course of the reaction and some interesting pharmaceutical compounds. Dissolving 5-bromo anthranillic acid in 100 ml of pyridine in o-amino benzoyl chloride stirring at room temperature for 30 minutes produce the cyclic compound 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one(1). The reaction of this compound with 75 mL of hydrazine hydrates for 3 hrs at 120-1300C. the reaction mixture was allowed to cool to room temperature to give 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one (2).
Materials and methods
Experimental
All reagents and solvents were purchased from sigma-Aldrich, in Germany. Melting points were determined on a kofler hot stage apparatus and were uncorrected. IR spectra were recorded on a Buck scientific IR M500 instrument. The 1H and 13C NMR spectra were recorded in DMSO-d6 at 400 MHz with HAZ VOLATILE V2. M Chemical shifts Sare reported in ppm relative to tetramethylsilane. Gas chromatography mass spectra were obtained on a Finingan MAT 44S mass spectrophotometer operating at 70eV. Elemental analysis agreed favourably with the calculated values. Analytical thin layer chromatography (TLC) was used to monitor the reactions.
Elemental Analysis
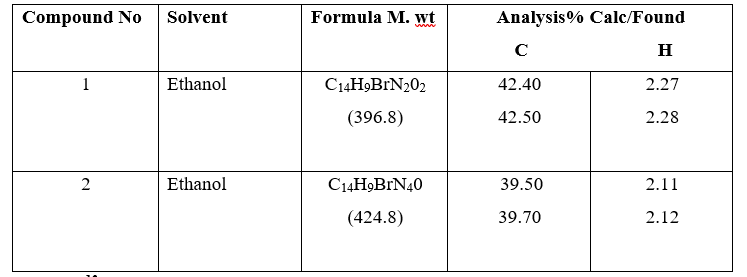
The compositions of the compounds are summarized in table 1. The C and H contents (both theoretically calculated values and actual values) are indicated.
Synthesis of 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one (1).
5-bromo anthranillic acid (.16M, 34.72gm) was dissolved in 100 ml of pyridine. To this reaction mixture o-amino benzoyl chloride (.16M, 24.8gm) was added with stirring at room temperature. Stirring continued for 30 mins at the same temperature. This reaction mixture was filtered out and collect the precipitate, which was washed with distilled water and Pet.ether 60/80 to remove the traces of pyridine. The pale creamish crystals obtained were dried at 600C. m.p.-1900C, yield- 75%,
Synthesis of 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one ( 2).
6-bromo-2-(o-aminophenyl)-3-,1-benzoxazin-4(3H)-one (0.075M, 23.775gm) was refluxed with 75 mL of hydrazine hydrate for 3 hrs at 120-1300C. the reaction mixture was allowed to cool to room temperature. Pale creamish crystals developed were recrystallized from super dry ethanol. m.p.-178-1800C, yield-75%,
Antimicrobial activities
Determination of zone of inhibition
The microbial growth inhibitory activities of the powdered crude drug obtained were determined by the agar well plate method where the compounds were initially dissolved in distilled water (1:1). Those compounds with activities were later tested at concentrations of 10, 15, 20, 60 mg/mL against clinical isolated Staphylococcus aureus, Bacillus species, Escherichia coli, Aspergillus Species, Klebsiella pneumonia, Pseudomonas Aeuriginosa and Candida albicans using the standard microbiological method. Sterile nutrient and Sabouraud dextrose agar plates were prepared for bacteria and fungi respectively and standardized inoculum of test organisms was spread uniformly.
We used a sterile borer (8 mm) and 100μL of the test concentrations, to bored six wells, standard antibiotic, and the solvent control were added to each well. The plates were left on the table for 1 h for the test solution to diffuse into the medium and then incubated at 37°C for 18-24 h. The resultant zone of inhibitions of microbial growth around the well was measured in mm. The test was performed in triplicate. Standard antibiotics ciprofloxacin (30 mg/mL), and Ketonaxol (50 mg/mL) were tested against bacteria and fungi respectively as the positive control [37].
Determination of MIC
The minimum inhibitory concentration (MIC) values of the powdered crude drug obtained were determined using the agar dilution method. Four different concentrations range of 100 µL of the synthesized compounds were incorporated into their respective molten agar and allowed to set. This was also repeated for ciprofloxacin and itraconazole as positive control and the diluent as a negative control. Each of the standardized test microorganisms was radially streaked onto the prepared plates. The plate was left to stand for 1 h at room temperature, incubated at 37°C for 18-24 h. The MIC was recorded as the lowest concentrations that inhibited the growth of each of the test organisms [37].
Results and Discussion
Table 1: Characterization AndPhysical Data Of Synthesized Compounds
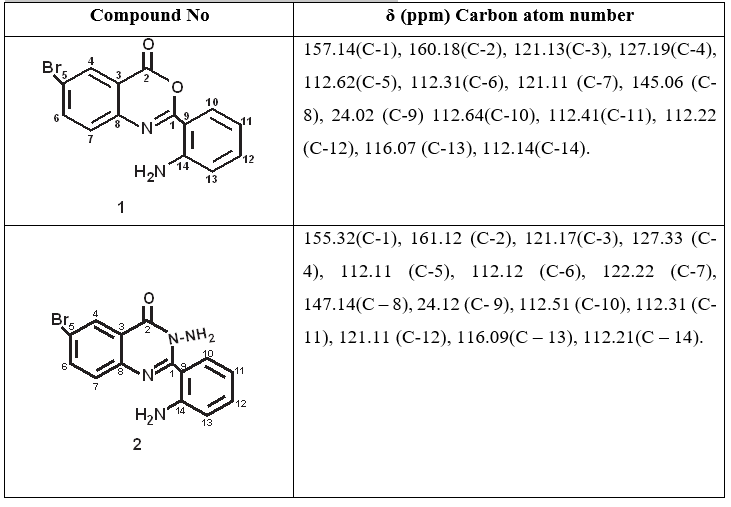
Table 2: 13C-NMR Of Synthesized Compounds
Table 3: 1H-NMR Of Synthesized Compounds
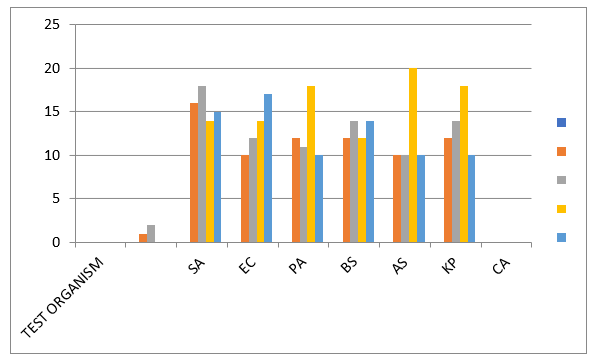
Figure 1: Antibacterial Activity of Control Drugs and Tested Synthesized Compounds Against Tested Standard Organism
Control drugs
Ciprofloxicin (CPX) for bacteria
Ketonaxol (PEF) for fungus
Compound 1 (1)
Compound 2 (2)
Figure 1: The effect of the Synthesized Compounds and Standard drugs toward studied bacteria. SA = Staphylococcus aureus, BS = Bacillus species, AS = Aspergillus Species, PA = Pseudomonas aeruginosa, EC = Escherichia coli, KP=Klebsiella pneumonia, and CA=candida albicans .
Significantly different from Ligand at P< 0>
Discussion
The present study reported the synthesis of two derivatives of quinazolinone, 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one( 1) and 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one ( 2) . The compounds were investigated for their Antibacterial activity.
Structural elucidations of compounds synthesized were characterized by correct elemental analysis and careful inspections of spectral data. Looking at the 1H NMR spectra of the compounds synthesized, compound 1 displayed a duplet at δ 5.64 which was due to amino, –NH2 group. Other duplet appeared at δ 7.18 and 7.25 attributed to aromatic protons. Also, 1H NMR spectrum of compound 2 showed a characteristic signal at δ 5.65 and 6.18 (singlet) corresponding to the two amino, –NH2 groups. Two singlets appeared at δ7.41 and 7.10 attributed to aromatic protons. Another signal appeared at 5.80 which is attributed to the protons of the amino group. For the IR spectra, compound 1 were characterized by the presence of 3068 υ C-H str. of the aromatic ring, 1698 cm-1 υ C=O str. of the ring, 3365 cm-1 3345 cm-1 υ N-H str. of the ring in the region of the compound. Compound 2 was characterized by presence of υ 3048 cm-1 υ (C-H str. of the aromatic ring), 3361 cm-1 , 3351 cm-1 υ (N-H str. of the ring), 1706 cm-1 υ (C=O str. of the ring), υ 1316 cm-1 region of the compound.
The 13C NMR spectrum of compound 1, revealed signals at δ24.02, attributed to phenyl group, while the aromatic carbon atoms appeared between δ values 112.31 – 160.18 with the carbonyl carbon atom appearing as the highest δ value of 160.18. Similarly, compound 2 showed signals at δ24.12, attributed to phenyl group, while the aromatic carbon atoms appeared between δ values 105.64 - 160.28, with the carbonyl carbon atom appearing as the highest δ value of 160.28.
These compounds synthesized exhibited promising Antibacterial activities. The antibacterial activity of compounds synthesized were determined using the agar well plate method and the results obtained are summarized in Figure 1. Compound 2 showed the highest activity against Staphylococcus aureus, Bacillus species, Escherichia coli, Klebsiella pneumonia compared to the other compound 1. It may be that the substitution of amino group at position three increases the activity. These compounds synthesized have a higher activity against Staphylococcus aureus, than Ciprofloxacin (CPX) and Ketonaxol (PEF), which are standard antibacterial drugs.
Conclusion
The present study has showed that the quinazolinone derivatives 1 and 2 have high antibacterial activity. Compound 2 showed the highest activity against Staphylococcus aureus, Bacillus species, Escherichia coli, Klebsiella pneumonia compared to the other compound 1. It may be that the substitution of amino group at position three increases the activity. These compounds synthesized have a higher activity against Staphylococcus aureus, than Ciprofloxacin (CPX) and Ketonaxol (PEF), which are standard antibacterial drugs. This study has confirmed that the antibacterial analysis shows that the compounds synthesized have high activity against Staphylococcus aureus, Bacillus species, Aspergillus Species, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumonia, with no activity against Candida albicans. From this result, Compound 2 could be a potential Antibacterial and a tool to pharmaceutical drug delivery.
Declarations
Conflict of interest
The author declares no conflict of interest.
Funding
No fund was obtained during the research.
Author declaration
The author hereby declares that the work presented in this article is original and that any liability for claims relating to the content of this article will be borne by me.
Ethics approval and consent to participate
Ethic approval, consent to participate and the procedure used was approved by the Ethic approval committee of Ondo State University of Science and Technology, Okitipupa, Ondo State, Nigeria.
Acknowledgements
The author acknowledges the assistance of Baba Haruna of the Department of Pharmaceutical Chemistry of Niger Delta University, Wilberforce Island, Yenogoa and Dr. Marvis, in England for running the spectra.
Declaration statement
The author declares there is no conflict of interest.
References
- Chandregowda V, Kush AK, Chandrasekara Reddy G: Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur J Med Chem. 2009, 44: 3046-3055. 10.1016/j.ejmech.2008.07.023.
View at Publisher | View at Google Scholar - Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Youssef KM, Al-Obaid AM, El-Subbagh HI: Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem. 2006, 14: 8608-8621. 10.1016/j.bmc.2006.08.030.
View at Publisher | View at Google Scholar - Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid LM, Neil JPO’, VanBrocklin HF: Synthesis of 6-acrylamido-4-(2-[18F] fluoroanilino) quinazoline: A prospective irreversible EGFR binding probe. J Lablelled Compd Rad. 2005, 48: 109-115. 10.1002/jlcr.903.
View at Publisher | View at Google Scholar - Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH: ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002, 62: 5749-5754.
View at Publisher | View at Google Scholar - Alagarsamy V, Solomon VR, Dhanabal K: Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H -quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg Med Chem. 2007, 15: 235-241. 10.1016/j.bmc.2006.09.065.
View at Publisher | View at Google Scholar - Baba A, Kawamura N, Makino H, Ohta Y, Taketomi S, Sohda T: Studies on disease-modifying antirheumatic drugs: synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect1. J Med Chem. 1996, 39: 5176-5182. 10.1021/jm9509408.
View at Publisher | View at Google Scholar - Rohini R, Muralidhar Reddy P, Shanker K, Hu A, Ravinder V: Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c]quinazolines. Eur J Med Chem. 2010, 45: 1200-1205. 10.1016/j.ejmech.2009.11.038.
View at Publisher | View at Google Scholar - Antipenko L, Karpenko A, Kovalenko S, Katsev A, Komarovska-Porokhnyavets E, Novikov V, Chekotilo A: Synthesis of new 2-thio-[1,2,4]triazolo[1,5-c]quinazoline derivatives and its antimicrobial activity. Chem Pharm Bull. 2009, 57: 580-585. 10.1248/cpb.57.580.
View at Publisher | View at Google Scholar - Jatav V, Kashaw S, Mishra P: Synthesis and antimicrobial activity of some new 3–[5-(4-substituted)phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med Chem Res. 2008, 17: 205-211. 10.1007/s00044-007-9054-3.
View at Publisher | View at Google Scholar - Aly AA: Synthesis of novel quinazoline derivatives as antimicrobial agents. Chin J Chem. 2003, 21: 339-346.
View at Publisher | View at Google Scholar - Li H, Huang R, Qiu D, Yang Z, Liu X, Ma J, Ma Z: Synthesis and bioactivity of 4-quinazoline oxime ethers. Prog Nat Sci. 1998, 8: 359-365.
View at Publisher | View at Google Scholar - Chandrika PM, Yakaiah T, Narsaiah B, Sridhar V, Venugopal G, Rao JV, Kumar KP, Murthy USN, Rao ARR: Synthesis leading to novel 2,4,6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell lines. Indian J Chem. 2009, 48B: 840-847.
View at Publisher | View at Google Scholar - Paneersalvam P, Raj T, Ishar PS M, Singh B, Sharma V, Rather BA: Anticonvulsant activity of Schiff bases of 3-amino-6,8-dibromo-2-phenyl-quinazolin-4(3H)-ones. Indian J Pharm Sci. 2010, 72: 375-378. 10.4103/0250-474X.70488.
View at Publisher | View at Google Scholar - Nandy P, Vishalakshi MT, Bhat AR: Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J Heterocycl Chem. 2006, 15: 293-294.
View at Publisher | View at Google Scholar - Saravanan G, Alagarsamy V, Prakash CR: Synthesis and evaluation of antioxidant activities of novel quinazoline derivatives. Int J Pharm Pharm Sci. 2010, 2: 83-86.
View at Publisher | View at Google Scholar - Lakhan R, Singh OP, Singh-J RL: Studies on 4 (3H)-quinazolinone derivatives as anti-malarials. J Indian Chem Soc. 1987, 64: 316-318.
View at Publisher | View at Google Scholar - Hess HJ, Cronin TH, Scriabine A: Antihypertensive 2-amino-4(3H)-quinazolinones. J Med Chem. 1968, 11: 130-136. 10.1021/jm00307a028.
View at Publisher | View at Google Scholar - Sasmal S, Balaji G, Kanna Reddy HR, Balasubrahmanyam D, Srinivas G, Kyasa S, Sasmal PK, Khanna I, Talwar R, Suresh J, Jadhav VP, Muzeeb S, Shashikumar D, Harinder Reddy K, Sebastian VJ, Frimurer TM, Rist Ø, Elster L, Högberg T: Design and optimization of quinazoline derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists. Bioorg Med Chem Lett. 2012, 22: 3157-3162. 10.1016/j.bmcl.2012.03.050.
View at Publisher | View at Google Scholar - Alvarado M, Barceló M, Carro L, Masaguer CF, Raviña E: Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem Biodivers. 2006, 3: 106-117. 10.1002/cbdv.200690001.
View at Publisher | View at Google Scholar - Malamas MS, Millen J: Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J Med Chem. 1991, 34: 1492-1503. 10.1021/jm00108a038.
View at Publisher | View at Google Scholar - P. Sharma, A. Kumar, P. Kumari, J. Singh, M.P. Kaushik, QSAR modeling of synthesized 3-(1,3-benzothiazol-2-yl) 2- phenyl quinazolin-4(3H)-ones as potent antibacterial agents, Med. Chem. Res. 21 (2012) 1136–1148.
View at Publisher | View at Google Scholar - X. Wang, J. Yin, L. Shi, G. Zhang, B. Song, Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one, Eur. J. Med. Chem. 77 (2014) 65–74. antimicrobial activities of some compounds, Chin. J. Org. Chem. 37 (2017) 385–393.
View at Publisher | View at Google Scholar - R. Bouley, D. Ding, Z. Peng, M. Bastian, E. Lastochkin, W. Song, M.A. Suckow, V.A. Schroeder, W.R. Wolter, S. Mobashery, M. Chang, Structure–activity relationship for the 4(3H)-quinazolinone antibacterials, J. Med. Chem. 59 (2016) 5011–5021.
View at Publisher | View at Google Scholar - X.H. Yang, X. Wang, M.H. Wu, Synthesis and biological properties of 3-(2-hydroxyethyl)-2-(phenylamino)quinazolin-4(3H)-ones, Chin. J. Org. Chem. 34 (2014) 1015–1020.
View at Publisher | View at Google Scholar - K.V. Vani, G. Ramesh, C.V. Rao, Synthesis of new triazole and oxadiazole derivatives of quinazolin-4(3H)-one and their antimicrobial activity, J. Heterocyclic Chem. 53 (2016) 719–726.
View at Publisher | View at Google Scholar - P. Asadi, G. Khodarahmi, A. Jahanian-Najafabadi, L. Saghaie, F. Hassanzadeha, Biologically active heterocyclic hybrids based on quinazolinone, benzofuran and imidazolium moieties: synthesis, characterization, cytotoxic and antibacterial evaluation, Chem. Biodiver. 14 (2017) e1600411.
View at Publisher | View at Google Scholar - F.W. Asker, S.A.R. Abbas, R.I. Al-Bayati, H.A. Al-Tamemi, Synthesis and biological evaluation of new quinazolinone derivatives, Eur. J. Chem. 5 (2014) 628–634.
View at Publisher | View at Google Scholar - M.K. Prashanth, H.D. Revanasiddappa, Synthesis of some new glutamine linked 2,3-disubstituted quinazolinone derivatives as potent antimicrobial and antioxidant agents, Med. Chem. Res. 22 (2013) 2665–2676.
View at Publisher | View at Google Scholar - M.S. Mohamed, M.M. Kamel, E.M.M. Kassem, N. Abotaleb, S.I.A. El-moez, M.F. Ahmed, Novel 6,8-dibromo-4(3H) quinazolinone derivatives of anti-bacterial and anti-fungal activities, Eur. J. Med. Chem. 45 (2010) 3311–3319.
View at Publisher | View at Google Scholar - Y.N. Mabkhot, M.S. Al-Har, A. Barakat, F.D. Aldawsari, A. Aldalbahi, Z. Ul-Haq, Synthesis, anti-microbial and molecular docking studies of quinazolin-4(3H)-one derivatives, Molecules 19 (2014) 8725–8739.
View at Publisher | View at Google Scholar - K.S. Kumar, S. Ganguly, R. Veerasamy, E.D. Clercq, Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4(3H)-ones, Eur. J. Med. Chem. 45 (2010) 5474–5479.
View at Publisher | View at Google Scholar - X. Gao, X. Cai, K. Yan, B. Song, L. Gao, Z. Chen, Synthesis and antiviral bioactivities of 2-aryl-or 2-methyl-3-(substituted- benzalamino)-4(3H)-quinazolinone derivatives, Molecules 12 (2007) 2621–2642.
View at Publisher | View at Google Scholar - S. Wang, M. Gao, G. Tan, H. Ma, Y. Zhao, H. Du, Z. Wang, H. Chen, X. Li, Effects of the substituent at C-2 phenyl on the N-/O-alkylation of quinazolin-4(3H)-one and anti-tumor, antimicrobial activities of some compounds, Chin. J. Org. Chem. 37 (2017) 385–393.
View at Publisher | View at Google Scholar - M. A. Mohamed, R.R. Ayyad, T.Z. Shawer, A.A.-M. Abdel- Aziz, A.S. El-Azab, Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds, Eur. J. Med. Chem. 112 (2016) 106–113.
View at Publisher | View at Google Scholar - N.A. Noureldin, H. Kothayer, E.-S.M. Lashine, M.M. Baraka, W. El-Eraky, S.A.E. Awdan, Synthesis, anticonvulsant activity, and SAR study of novel 4-quinazolinone derivatives, Arch. Pharm. Chem. Life Sci. 350 (2017) e1600332.
View at Publisher | View at Google Scholar - HaseenaBanu B., Bharathi, K. and Prasad, KVSRG. Synthesis, characterization and evaluation of in vitro antioxidant and anti-inflammatory activity of 2- (4-oxo-2-phenylquinazoline-3 (4H)-yl) substituted acetic acids. IOSR Journal of Pharmacy. (2002); Vol. 2: Issue 1pp 097-104.
View at Publisher | View at Google Scholar - Peter, O. O., Mary, O. E., and Cyril, O. U., 2021. Synthesis and antibacterial activities of quinazolin-4(3h)-one, 2-methyl-4(3h)- quinazolinone and 2–phenyl-4(3h)-quinazolinone. International Journal of Biological and Pharmaceutical Sciences Archive, 2021, 01(02), 077–084.
View at Publisher | View at Google Scholar