Research Article | DOI: https://doi.org/10.58489/2836-502X/007
Synthesis and Analgesic Activity of 3-(3-methoxyphenyl)-2-methylsulfanyl-3Hquinazolin-4-one (4) and 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro1H-quinazolin-4-one (3) Via N-(3- methoxyphenyl)-methyl dithiocarbamic acid (2).
- Osarumwense Peter Osarodion 1
- Department of Chemical Science, Ondo State University of Science and Technology, Okitipupa, Ondo State, Nigeria.
*Corresponding Author: Osarumwense Peter Osarodion
Citation: Osarumwense Peter Osarodion, (2023). Synthesis and Analgesic Activity of 3-(3-methoxyphenyl)-2-methylsulfanyl-3Hquinazolin-4-one (4) and 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro1H-quinazolin-4-one (3) Via N-(3- methoxyphenyl)-methyl dithiocarbamic acid (2). Journal of Endocrine System and Diabetes. 2(1). DOI: 10.58489/2836-502X/007.
Copyright: © 2023 Osarumwense Peter Osarodion, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 28 January 2023 | Accepted: 03 February 2023 | Published: 06 February 2023
Keywords: analgesic activity, quinazolinone, 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro1h-quinazolin-4-one, n-(3- methoxyphenyl)-methyl dithiocarbamic acid, 3-(3-methoxyphenyl)-2-methylsulfanyl-3hquinazolin-4-one.
Abstract
Introduction: 4(3H)-quinazolinone rings have been reported to possess different biological activities such as antibacterial, antifungal, antitubercular, antiviral, anticancer. These activities also include antihypertensive, diuretic, antimicrobial, pesticidal, anticonvulsant, anaesthetic and sedative activities, anti-malarial, and anti-diabetic. Methods/Experimental: The compound, 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro1H-quinazolin-4-one (3) was synthesized by dissolving Methyl anthranilate and N-(3- methoxyphenyl)-methyl dithiocarbamic acid in ethanol and anhydrous potassium carbonate and refluxed for 23 h and re-precipitated by treating with dilute hydrochloric. When tested for their in vitro analgesic activity using acetic acid induced induced writhing in mice, the compounds had Analgesic activity. Result: The compounds exhibited significant analgesic activity in the range of 74.67 - 83.80% in comparison to control. Conclusions: From our findings, the compounds synthesized have higher analgesic activities as compared to the standard analgesic drug.
Introduction
Quinazolinones and quinazolines are noteworthy in medicinal chemistry, because of wide range of their antibacterial, antifungal [1,2,3,4,5.6], antiinflammatory [7,8], antimalarial [9], anti-HIV [10], antiviral [10,11], antituberculosis [1, 12], (1,12) properties and also their inhibitory effects on thymidylate synthase [1,2,3,4,5.6], poly-(ADP-ribose) polymerase (PARP) [15, 16, 17], and thyrosine kinase [18, 19]. There are several approved drugs with quinazoline structure in the market such as, prazosin hydrochloride, doxazosine mesylate and terazosine hydrochloride [20, 21].
In the early 1900s, Paul Ehrlich, the legendary German chemist, initiated the use of drugs for infectious diseases. He developed methods for screening a series of chemicals for their potential activity against diseases. The term ‘‘chemotherapy’’, which means the use of chemicals to treat disease, was also coined by him [22].
The synthetic drugs were hugely used in early twentieth century (1900-1930s). But the use of synthetic drugs for treating microbial diseases reduced after the discovery and development of antibiotics. A paradigm shift in therapeutics for treating bacterial diseases took place after the industrial production of penicillin and succeeding development of other antibiotics. There was extraordinary decline in encumber of disease due to large-scale use of these antibiotics [23].
Hence, a general opinion was generated among citizens and policy-makers that infectious diseases would not produce significant problem in the future. But to everyone’s surprise, in the last few decades the historical statement, made by the surgeon-general William H. Stewart in the US Congress (1969)- “It is time to close the book on infectious diseases”, has not only been reversed but left least possibility of the closure of the said book [24].
These findings prompted the author to synthesis these quinazolinone derivatives with the aim of obtaining more precise information about the course of the reaction and determine the Analgesic properties.
Materials and Methods
General Experimental Procedure
The whole reagent and solvent that were used for the study were bought from sigma-Aldrich chemical company in Germany. Melting points were established using the Kefler hot stage apparatus and were not alter. The Buck scientific IR M500 instrument was used for the recording of the IR spectra. The 1H and 13 C N M R spectra were recorded in D M S O at 400 MHz with HAZ VOLATILE V2.M. As generally known, chemical shifts are reported in ppm relative to tetramethylsilane. Gas chromatography Mass (GC/MS) spectra were obtained on a Finingem MAT 44S mass spectrometer operating at electron impact energy of 70eV. Elemental analysis data were fully related to the calculated values. Analytical Thin Layer Chromatography (TLC) was used to monitor the reactions.
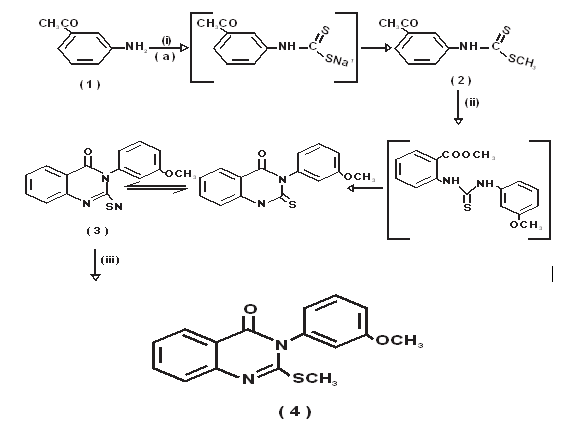
Scheme 1
i = CS2/NaOH
a = DMSO
ii = Methyl Anthranilate / EtOH, ∆
iii = NaOH / EtOH, (CH3)2SO4
Synthesis of 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro1H-quinazolin-4-one (3)
A solution of 3-methoxy aniline 1 (0.02 mol) in dimethyl sulphoxide (10 mL) was stirred vigorously. To this solution carbon disulphide (1.6 mL; 0.026 mol) was added and aqueous sodium hydroxide 1.2 mL (20 molar solution) was added drop wise during 30 min with stirring. Dimethyl sulphate (0.02 mol) was added gradually keeping the reaction mixture stirring in freezing mixture for 2 h. The reaction mixture was then poured into ice water. The solid obtained was filtered, washed with water, dried and recrystallized from ethanol. Methyl anthranilate (0.01 mol) and the above prepared N-(3- methoxyphenyl)-methyl dithiocarbamic acid (0.01 mol), were dissolved in ethanol. To this, anhydrous potassium carbonate was added and refluxed for 23 h. The reaction mixture was cooled in ice and the solid separated was filtered and purified by dissolving in 10% alcoholic sodium hydroxide solution and re-precipitated by treating with dilute hydrochloric acid. The solid obtained was filtered, washed with water, dried and recrystallized from ethanol. Yield = 86 %, mp 256-257 °C. IR: 3311 (NH), 1691 (C=O), 1211 (C=S) cm-1. 1 H NMR (CDCl3): 3.10 (s, 3H, OCH3), 7.30-7.91 (m, 8H, ArH), 10.52 (br s, 1H, NH); MS (m/z): 284 [M+ ].
Synthesis of 3-(3-methoxyphenyl)-2-methylsulfanyl-3Hquinazolin-4-one (4)
The 3-(3-methoxyphenyl)-2-thioxo-2,3-dihydro-1Hquinazolin-4-one 4 (0.01 mol) was dissolved in 40 mL of 2% alcoholic sodium hydroxide solution. To this dimethyl sulphate (0.01 mol) was added drop wise with stirring. The stirring was continued for 1 h, the reaction mixture was then poured into ice water. The solid obtained was filtered, washed with water, dried and recrystallized from ethanolchloroform (75:25) mixture. Yield = 86%, mp 155-156 °C; IR: 1690 (C=O) cm-1; 1 H NMR (CDCl3): 2.85 (s, 3H, SCH3), 3.34 (s, 3H, OCH3), 7.23-7.72 (m, 8H ArH); MS (m/z): 298 [M+]; Anal. Calcd. for C16H14N2O2S: C, 64.41; H, 4.72; N, 9.38. Found: C, 64.45; H, 4.74; N, 9.33.
Pharmacological evaluation
Swiss mice (30 - 40 g) of both sexes were used. The animals were maintained under standard diet and water. Test compounds were administered orally at dose levels. Ethic approval of animal use was obtained from Ethics committee of the Faculty of pharmacy, University of Benin, Benin City Nigeria.
Acetylsalicylic acid (100 mg/kg) was used as standard in the analgesic assay. There was a dose dependent decrease in writhing which was significant (p < 0>
Analgesic activity
The acetic acid induced abdominal constriction method is widely used for the evaluation of peripheral antinociceptive activity [21]. Swiss albino mice (30 – 40 g) were divided into five groups of 5 animals per group of both sexes (pregnant females excluded) and were given a dose of a test compound. Animals in group I received distilled water per oral to serve as control. Group II, III and IV were administered the compounds at doses of 100 mg/kg body weight respectively per oral. Group V animals were treated with acetylsalicylic acid (100 mg/kg body weight) by same route. After one hour of treatment, animals were administered 0.6
Results and Discussion
Table 1: Characterization and physical data of synthesized compounds
Compound No | Solvent | Formula M. wt | Analysis Calc/Found C H | |
3 | Ethanol | C15H12N202S (284) | 62.20 62.10 | 5.18 4.98 |
4 | Ethanol | C16H14N2O2S (298) | 64.41 64.40 | 4.73 4.71 |
Table 2: 1H-NMR of Synthesized compounds
Table 3: 13C-NMR of Synthesized compounds
Table 5: Effect of the test compounds on acetic acid induced writhing in mice.
Compound No | Does mg/kg (p.o) | Numbers of writhing (per 20 min) | % Inhibition
|
3 | 20 40 | 47.41 + 0.11 32.40+ 0.22 | 35.78 57.54 |
4 | 20 40 | 31.05+ 2.14 22.16+ 0.15 | 59.49 72.38 |
TWEEN 80 | 0.2ML | 69.00 + 0.12
|
|
Acetylsalicylic acid |
| 22.50 + 3.07 | 67.39 |
Indomethacin | 10 | 14.80 + 4.95 | 78.55 |
Values are meant + S.E.M; P 0.001, significantly different from control, paired t-test (n=5), P.O = per oral.
Discussion
Synthetic route depicted in Scheme (1) outline the chemistry part of the present work. The key intermediate 3-(3- methoxyphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one (4) was obtained by reacting 3-methoxy aniline (1) with carbon disulphide and sodium hydroxide in dimethyl sulphoxide to give sodium dithiocarbamate, which was methylated with dimethyl sulfate to afford the dithiocarbamic acid methyl ester (2). Compound 2 on reflux with methyl anthranilate (3) in ethanol yielded the desired 3-(3- methoxyphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one (4) via the thiourea intermediate in good yield (82%).
The synthesized compounds were screened for their in vitro antibacterial activity against Staphylococcus aureus, Bacillus species, Escherichia coli, Klebsiella pneumonia, Enterococcus Feacalis, Pseudomonas aeriginosa and Candida albicans. The results of antibacterial activity depicted in Table. 1 indicates that the test compounds inhibited the growth of the bacterial in varying degree. Compounds with proton substituents to the sulphur showed higher antibacterial activity over the methyl substituents to sulphur.
Structural elucidations of compounds synthesized were characterized by correct elemental analysis and careful inspections of spectral data. Looking at the 1H NMR spectra of the compounds synthesized, compound 3 displayed a singlet signal at: δ 3.10 attributed to methoxy group. Other singlet appeared at δ7.30 and 7.91 attributed to aromatic protons. Two singlet appeared at δ7.41 and 7.10 attributed to aromatic protons. Another signal appeared at 10.52 which were attributed to the protons of the amino group.
The 13C NMR spectrum of compound 3, revealed signals at δ51.92 attributed to the methoxy group, while the aromatic carbon atoms appeared between δ values 100.04 -168.27 with the carbonyl carbon atom appearing as the highest δ value of 1691.01. Similarly, compound 4 showed signals at δ22.57, and 56.81 attributed to methyl and the methoxy groups respectively, while the aromatic carbon atoms appeared between δ values 105.65-160.26, with the carbonyl carbon atom appearing as the highest δ value of 169.02.
The 13C nuclear magnetic resonance revealed low δ values for the aliphatic carbons. This is because the alkyl group is electron donating and hence produces a shielding effect which makes the carbon atom to resonate at low δ values. The aromatic and the carbonyl carbon atoms appeared at high δ values. This is because the aromatic ring is electron withdrawing and the aromatic carbons are highly deshielded and resonate at high frequency. The electronegative effect of the oxygen atom on the carbonyl group makes the carbonyl carbon to appear at higher δ value.
The compounds were investigated for their analgesic activity. The compounds synthesized exhibited promising analgesic activity In addition, compound 3 showed higher activity than compound 4. Table 3
Conclusions
In summary, synthesis of new series of 1-(4-oxo-3-(3- methoxyphenyl)-3H-dihydroquinazolin-2-yl)-4-(substituted) thiosemicarbazides has been described. These derivatives have exhibited significant analgesic activity. The compounds exhibited the analgesic activity and offers potential for further optimization and development to new antitubercular agents.
The present study has showed that the quinazolinone derivatives 3 and 4 have analgesic activity. Compounds 3 showed higher activity compared to the control drugs, indomethacin and acetylsalicyclic acid, which is a standard analgesic drug.
Acknowledgements: The author appreciate the assistance of Dr. Marvis E, in England for running the spectra and the Department of Pharmacology, Faculty of Pharmacy, University of Benin for providing the test animals.
Conflicts of interest: The author declares no conflict of interest.
Authors’ declaration: The author hereby declares that the work presented in this article is original and that any liability for claims relating to the content of this article will be borne by me.
References
- Samira I, Patel S, Hasmin M, Patel S. (2012) Biological profile of quinazoline. Int J Pharm Chem Sci. 2012; 1:1863–1872.
View at Publisher | View at Google Scholar - Singh VK, Singh SK, Gangwar L. (2013) Synthesis and antimicrobial activity of novel fused 4-(3H) quinazolinone derivatives. Int J Sci Res. 2013; 2:425–428.
View at Publisher | View at Google Scholar - Ghorab MM, Ismail Z, Radwan AA, Abdalla M. (2013) Synthesis and pharmacophore modeling of novel quinazolines bearing a biologically active sulfonamide moiety. Acta Pharm. 2013; 63:1–18.
View at Publisher | View at Google Scholar - Vijayakumar K, Ahamed AJ, Thiruneelakandan G. (2013) Synthesis, antimicrobial, and anti-HIV1 activity of quinazoline-4(3H)-one derivatives. J Applied Chem 2013. 2013 ID 387191.
View at Publisher | View at Google Scholar - Deep A, Narasimhan B, Ramasamy K, Mani V, Mishra RK, Majeed AB. (2013) Synthesis, antimicrobial, anticancer evaluation and QSAR studies of thiazolidin-4-ones clubbed with quinazolinone. Curr Top Med Chem. 2013; 13:2034–2046.
View at Publisher | View at Google Scholar - Al-Amiery AA, Kadhum AAH, Shamel M, Satar M, Khalid Y, Mohamad AB. (2014) Antioxidant and antimicrobial activities of novel quinazolinones. Med Chem Res. 2014; 23:236–242.
View at Publisher | View at Google Scholar - Laddha SS, Wadod Kar SG, Meghal SK. (2006) Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory, antimicrobial activity of 6,8-disubstituted 2-phenyl-3- [substituted- benzothiazol-2-yl]-4(3H)-quinazolinone. Arkivoc. 2006; 11:1–20.
View at Publisher | View at Google Scholar - Giri RS, Thaker HM, Giordano T, Williams J, Rogers D, Sudersanam V, et al. (2009) Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazolin-4-one derivat-ives as inhibitors of NF-ĸB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur J Med Chem. 2009; 44:2184–2189.
View at Publisher | View at Google Scholar - Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, et al. (2005) Antimalaria activities and therapeutic properties of febrifugine analogs. Antimicrob Agents Chemoter. 2005; 49:1169–1176.
View at Publisher | View at Google Scholar - Deetz MJ, Malerich JP, Beatty AM, Smith BD. (2001) One-step synthesis of 4(3H) quinazolinones. Tetrahedron Lett. 2001; 42:1851–1854.
View at Publisher | View at Google Scholar - Krishnan SK, Ganguly S, Veeramy R, Jan B. (2011) Synthesis, antiviral and cytotoxic investigation of 2-phenyl-3-substituted quinazolin-4(3H)-ones. Eur Rev Med Pharmacol Sci. 2011; 15:673–681.
View at Publisher | View at Google Scholar - Khosropour AR, Mohammadpoor-Baltork I, Ghorbankhani H. (2006) Bi (TFA) 3-[nbp] Fecl4: A new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives. Tetrahedron Lett. 2006; 47:3561–3564.
View at Publisher | View at Google Scholar - Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AAM, Abdel-hamide SG, et al. (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem. 2006; 14:8608–8621.
View at Publisher | View at Google Scholar - Liu S, Liu F, Yu X, Ding G, Xu P, Cao J, et al. (2006) The 3D-QSAR analysis of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains on thymidylate synthase. Bioorg Med Chem. 2006; 14:1425–1430.
View at Publisher | View at Google Scholar - Hattori K, Kido Y, Yamamoto H, Ishida J, Iwashita A, Mihara K. (2007) Rational design of conformationally restricted quinazolinone inhibitors of poly (ADP-ribose) polymerase. Bioorg Med Chem Lett. 2007; 17:5577–5581.
View at Publisher | View at Google Scholar - Guiles J, Sun X, Critchley IA, Ochsner U, Tregay M, Stone K, et al. (2009) Quinazolin-2-ylamino-quinazolin-4-ols as novel non-nucleoside inhibitors of bacterial DNA polymerase III. Bioorg Med Chem Lett. 2009; 19:800–802.
View at Publisher | View at Google Scholar - Orvieto F, Branca D, Giomini C, Jones P, Koch U, Ontoria JM, et al. (2009) Identification of substituted pyrazolo [1,5-a] quinazolin-5 (4H)-one as potent poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors. Bioorg Med Chem Lett. 2009; 19:4196–4200.
View at Publisher | View at Google Scholar - Khalil AA, Abdel hamide SG, Al-Obaid AM, El-subbagh H. (2003) Substituted quinazolinones, Part2.Synthesis and in vitro anticancer evaluation of new 2-substituted mercapto-3H-quinazolin analogs. Arch Pharm Pharm Med Chem. 2003; 336:95–103.
View at Publisher | View at Google Scholar - Nagar AA, Rathi LG, Chugh N, Pise VJ, Bendale A. (2010) Microwave assisted one pot synthesis of 2, 3-di-substituted quinazolin-4- (3H)-ones and their potential biological activity. Der Pharm Chem. 2010; 2:37–43.
View at Publisher | View at Google Scholar - Selvam TP, Kumar PV. (2011) Quinazoline Marketed drugs - A Review. Res Pharm. 2011; 1:1–21.
View at Publisher | View at Google Scholar - Abida, Parvez N, Rana A, Imran M. (2011) An updated review: newer quinazoline derivatives under clinical trial. Int J Pharm Biol Arch. 2011; 2:1651–1657.
View at Publisher | View at Google Scholar - DeVitaJr VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68: 8643–8653.
View at Publisher | View at Google Scholar - Davies J, Davies D (2010) Origins and Evolution of Antibiotic Resistance.MicrobiolMolBiol Rev 74: 417–433.
View at Publisher | View at Google Scholar - Spellberg B, Taylor-Blake B (2013) On the exoneration of Dr. William H. Stewart: debunking an urban legend.Infect Dis Poverty 2: 3.
View at Publisher | View at Google Scholar