Review Article | DOI: https://doi.org/10.58489/2836-2411/028
Surgical Site Infections in Diabetic Patients: literature review
- Zeinab Kazan 1,3
- Ahmad Saifan 1,3
- Jimmy Dagher 2,3
- Fatima Mroueh 1,3
- Ahmad Afyouni 1,3
- Karim Hamideh 1
- Layal Msheik 1,3*
- Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
- School of Medicine and Medical Sciences, Holy Spirit University of Kaslik
- Medical Learning Skills Academy, Beirut, Lebanon
*Corresponding Author: Layal Msheik
Citation: Zeinab Kazan, Ahmad Saifan, Jimmy Dagher, Fatima Mroueh, Ahmad Afyouni, Karim Hamideh, Layal Msheik. (2023). Surgical Site Infections in Diabetic Patients: literature review. Journal of Internal Medicine and Health Affairs. 2(4); DOI: 10.58489/2836-2411/028
Copyright: © 2023 Layal Msheik, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 12 September 2023 | Accepted: 16 October 2023 | Published: 18 October 2023
Keywords: Diabetes mellitus, Wound healing, Surgical Site Infections (SSI), Risk factors, Bacterial pathogens, Biomarkers, Complications, Management
Abstract
Diabetes mellitus is a chronic metabolic condition with severe repercussions in surgical treatment. One important component of this effect is the complex link between diabetes and Surgical Site Infections (SSI). Diabetes severely slows wound healing, owing to poor microvascular circulation, decreased collagen production, and diminished immunological responses. Diabetic patients are more likely to develop SSI as a result of variables such as hyperglycemia, neuropathy, obesity, and peripheral vascular disease. Staphylococcus aureus, Escherichia coli, and Enterococcus species are common bacterial infections that cause SSI in diabetics. Furthermore, this review emphasizes important biomarkers, such as C-reactive protein and procalcitonin, which provide indications for SSI in diabetics. Deep tissue involvement, sepsis, and extended hospitalization are among the consequences associated with these infections, which need quick and customized care measures. By focusing on both diabetes control and SSI prevention, healthcare practitioners may reduce the significant burden both conditions place on patients and the healthcare system, thereby improving outcomes for diabetic patients having surgical operations.
Introduction
Diabetes Mellitus (DM) is characterized by uncontrolled glucose levels caused by insulin deficiency, malfunction, or both, culminating in hyperglycemia [1]. Globally, one in every eleven persons has diabetes [2], and the prevalence is expected to rise from 415 to 642 million by 2040, particularly in low-income countries [3]. Diabetes mellitus (DM) is classified into two types: type 1 (T1DM) and type 2 (T2DM) [2]. T1DM is an autoimmune illness in which the body's immune system wrongly attacks and kills the pancreas' insulin-producing beta cells. T2DM, on the other hand, is largely defined by insulin resistance, which occurs when the body's cells do not respond adequately to insulin [1]. Diabetes is characterized by hyperglycemia, which is also the root cause of its complications and consequences on other systems. Chronic and persistent hyperglycemia has a poor prognosis and raises the risk of potentially fatal consequences [2]. Surgical site infection (SSI) is one of the most serious consequences of preoperative and intraoperative hyperglycemia [4]. Prior to the introduction of antiseptics and other sanitary procedures, SSI mortality was quite high [5]. SSI is defined as superficial incisional infection, which affects just the skin and subcutaneous tissue, deep incisional infection, which affects deeper tissues, and organ/space infection, which affects any organ other than the incision site but must be connected to the surgical process [6]. Preexisting infection, malnutrition, low serum albumin, the elderly, smoking, and immunosuppression (diabetes mellitus, irradiation) have all been identified as key risk factors for this SSI [7]. (Olsen et al, 2008) found that preoperative blood glucose levels more than 125 mg/dL or postoperative serum glucose levels greater than 200 mg/dL were independent causes for SSI after spine surgery [8]. Diabetes contributes to SSI because it promotes neuropathy, vascular damage, and cellular malfunction, particularly in immune cells, and decreases immunity [9]. Bacteria that invade surgical wounds can be gram-positive or gram-negative [10]. Staphylococcus aureus was the most frequent isolate among Gram-positive bacteria, followed by coagulase-negative Staphylococcus, while Escherichia coli, Klebsiella species, and Citrobacter freundii were the most prevalent among Gram-negative bacteria [11]. This review will highlight the effect of diabetes on wound healing, risk factors contributing to SSI in diabetic patients and most common bacteria isolated from these infections, important biomarkers indicating the presence of SSI in diabetics, complications of these SSI, and their management.
Discussion
1. Physiology of Wound Healing and Its Disruption in Diabetic Patients
a. Normal wound healing:
After a tissue lesion forms, a series of molecular and cellular processes occur in an attempt to restore the wounded tissue [12]. Sequential processes involving soluble mediators, blood cells, and parenchymal cells are incorporated during the exudative, proliferative, and extracellular matrix remodeling phases [13]. Vasoconstriction begins immediately after damage, followed by platelet coagulation to create a clot that protects the lesion and prevents additional bleeding [14]. This is followed by an inflammatory phase, which inhibits inflammatory cell movement to clear debris and activate other activities [13]. The most significant cells are macrophages, which stimulate the recruitment of other cells, mostly leukocytes, by releasing various cytokines. Because of their phagocytic ability, macrophages eliminate excess apoptotic particles, lowering inflammation while stimulating other cells to act [12]. The inflammatory phase is followed by the proliferative phase, which is characterized by the development of granulation tissue and the regeneration of the vascular network. [14]. The fibroblasts are the main cells in this process, which begins after 2 to 3 days [12]. Cells begin to proliferate, primarily fibroblasts and endothelial cells, and angiogenesis develops to reestablish blood flow and feed the tissue. At the same time, stem cells differentiate and keratinocytes proliferate, resulting in epithelization [14]. The healing process then progresses to the remodeling stage [13]. During this stage, the previously produced granulation tissue is replaced by permanent scar, which requires a perfect balance between new tissue synthesis and breakdown that must be meticulously maintained [12,14].
b. Effect of diabetes and hyperglycemia on wound healing:
Diabetes delays the healing process, resulting in a non-healing wound that can lead to a number of complications [15]. Diabetes, especially hyperglycemia, causes endothelial and fibroblast dysfunction, free radical damage, immune system dysfunction, neuropathy, reduced angiogenesis, and poor granulation tissue growth [15,16,17]. Hyperglycemia causes atherosclerosis, which decreases nutrients and blood supply to the regenerating tissue, causing healing to be slowed [16]. Endothelial and fibroblast dysfunction are also significant effects [16,17]. Functional fibroblasts are required for optimal and controlled wound healing because they secrete growth factors, secrete, contract, and change the extracellular matrix. As a result, any defect in these cells will impede wound healing [17]. Furthermore, a decrease in IGF1, a potent chemotactic agent, inhibits these cells' chemotaxis [15]. Poor wound healing is exacerbated by free radical damage and oxidative stress [18]. One cause of free radical damage is decreased activity of antioxidant enzymes such as glutathione peroxidase and superoxide dismutase [19]. Oxidative damage, in conjunction with hyperglycemia, causes neuron ischemia and sensory, motor, and autonomic neuropathy [15]. Because neuropathic skin contains fewer neurons, it promotes poor and prolonged wound healing [20]. Sensory neuropathy results in loss of pain perception [15], whereas autonomic neuropathy results in decreased sweat gland activity, resulting in dry skin and pruritus, as well as delayed wound healing and increased risk of infections [18]. The formation of granulation tissue in diabetics lacks a typical cascade of events that defines healthy wound healing [16]. The platelet-derived growth factor is required for fibroblast recruitment and expansion, the creation of granulation tissue proteins and temporary extracellular matrix, and angiogenesis throughout the healing process. In diabetic wounds, the expression of PDGF and its receptors is decreased, indicating a malfunction in the healing process [15]. Diabetes also modifies the angiogenic program, resulting in an unanticipated unequal distribution of soluble angiogenic factors [16]. It also has an effect on immune system reaction [15]. (Mallik et al., 2018) found that hyperglycemia and oxidative stress affect macrophage epigenetic coding, generating changes in macrophage polarization and modulation, resulting in dysregulation and a longer recovery period [21]. As previously stated, diabetic individuals have poor chemotaxis, which inhibits the migration of various inflammatory cells and causes these cells to malfunction, resulting in delayed wound healing and an increased risk of infection [15].
2. Risk factors contributing to infections in diabetic patients
a. Neuropathy and Reduced Sensation
Within 25 years of the onset of diabetes, approximately half of patients establish peripheral neuropathy [22]. This complication is determined by the clinical manifestations of peripheral nerve damage in patients with diabetes after ruling out other causes of neuropathy that are categorized either as sensory, motor, or autonomic in accordance with the type of nerve fibers attained. Hyperglycemia is the main cause behind neuropathy triggering a series of events comprising increased polyol pathway and hexosamine influx, building up of glycosylation end products, stimulation of protein kinase C (PKC), and oxidative stress [23]. As a result of the structural nerve damage, the risk of trauma is greatly increased. Extremities infections, wounds, and ulcerations are underestimated due to the absence of pain symptomatology [24]. As does continuous painless injury caused by neuropathy, enhances the risk of extremity infections [25]. Diabetic patients who have already lost their protective sensation reported significantly higher rates of postoperative infections [26].
b. Vascular Complications and Impaired Blood Flow
Correspondingly, neuropathy due to endoneurial microvascular damage is a form of vascular complication in diabetes. Diabetic patients had higher arterial stiffness and reported vascular endothelial cell malfunction [27]. Diabetes-related metabolic disorders, including hyperglycemia, insulin resistance, and free fatty acids, may activate numerous mechanisms that modify the blood vessels’ structure and functionality. Certainly, mechanisms that may cause or worsen vascular damage, including the inactivation of nitric oxide, mitochondrial dysfunction, the activation of PKC, increased apoptosis caused by elevated oxidative stress, the formation of reactive oxygen species secondary to different biochemical pathways either the polyol pathway or the accumulation of advanced glycation end products (AGEs) [27,28]. Furthermore, the fundamental hallmarks of diabetic vasculopathy are changes in vascular homeostasis caused by endothelial and vascular smooth muscle cell dysfunction, which promotes a pro-inflammatory/thrombotic state and eventually causes atherothrombosis. Diabetes-related macro- and microvascular problems are mostly caused by chronic hyperglycemia [28]. All these mechanisms and pathways can lead to reduced blood circulation and perfusion of extremities, hindering the delivery of immune cells and nutrients to the wound site thus increasing the risk of infections [15].
c. Immunodeficiency and Impaired Immune Response
In normal circumstances, the human body employs incredible processes to defend itself against countless infectious agents. Pathogens find it difficult to penetrate this protective mechanism under normal settings, but a variety of situations and flaws cause the immune system to malfunction. Unfortunately, diabetes is one of those situations that might disrupt the host's immune system [15,29]. Insulin insufficiency and hyperglycemia can impact cellular immunity in addition to the danger caused by the natural barrier degradation resulting from neuropathy [29]. Diabetes and hyperglycemia have a wide range of adverse effects on immunological defense mechanisms, either cellular or humoral. These effects include cytokine production impairment, inhibition of leukocyte recruitment, flaws in pathogen recognition, and alteration in leukocyte functionality [29,30]. Diabetes causes suppression of numerous cytokine secretions, like IL-2, IL-6, IL-10, and IL-22, [29] especially IL-6 because of its importance in the adaptive immune response by increasing antibody secretion and effector T-cell growth. These findings suggest that the lack of those cytokines might decrease the immunological response against invading pathogens in diabetic patients [31]. These changes weaken innate immunity, considered as the first line of defense [32]. In the same way, adaptive immunity is also altered, including reduced T cell activity, elevated inflammatory T helper characteristics, reduced regulatory T cells [33], and hindered B cell functionality [34]. These changes are harmful to the host because they affect tissue healing, enhance inflammatory response, disrupt regulation, and cause an aberrant humoral response causing the patient to have weak immunity against pathogens and increase the risk of infections. [15,32]
d. Obesity and Increased Skin Folds
Obesity, previously thought as an isolated problem has changed over the last several decades and is now recognized as a systemic disease involving various organs and systems, especially what is known as the largest organ, the skin [35]. Obese patients' skin is associated with a great number of physiological changes, characterized by more subcutaneous fat, greater skin folds, and more roughness on the surface [36]. This causes a decrease in collagen synthesis relative to the skin surface area reducing the rigidity and structural integrity of the skin itself, in addition to an increase in the water permeability of the skin leading to drier skin [37]. Adipose tissue also acts as a very effective insulator by retaining heat, raising core temperatures, and producing excessive perspiration. Increased moisture, particularly in skin folds, encourages fungal or bacterial growth [37,38]. Hyperhidrosis is established, presumably due to impaired temperature control, a rise in sweat gland activities directly correlated to the increase of skin moisture [37]. The sweating and friction within skin folds can lead to Intertrigo, an inflammatory condition, associated with microorganisms’ overgrowth, most regularly with Candida or Gram-positive bacteria [38,39]. Likewise, erythrasma is another skin fold infection due to Corynebacterium colonization [36,39]. The diversity of the skin's microbiome fluctuates and is mostly affected by the physiological condition of the cutaneous sites, which is certainly influenced by moisture, dryness, sebum secretion, and temperature [39].
3. Most common types of bacteria causing SSI
a. S. Aureus:
Staphylococcus Aureus is the most common type of surgical site infection (SSI) accounting for 30.4% of all SSIs [40]. Knowing that diabetes can be considered an independent risk factor for SSIs for multiple surgical procedure types [4]. Consequently, diabetic patients will be more susceptible to S. Aureus infections in SSIs as it is the most common bacterium affecting soft tissues in diabetics [41]. Moreover, Methicillin-resistant S. Aureus (MRSA) accounts for approximately half of the S. Aureus SSIs [42]. Due to the fact that diabetes mellitus is a well-recognized risk factor for hospital-acquired MRSA infections [43], it can be established that MRSA SSIs increased in diabetic patients too.
b. E. Coli:
Escherichia Coli accounts is also one of the common bacteria isolated in SSI [40]. Nonetheless, (Alkaaki et al., 2019) showed that, including only abdominal surgical procedures, E. Coli was the commonest SSI causative agent counting 26 out of 50 patients. In this study, patients with diabetes had a higher rate of infection [10]. Furthermore, the presence of enteric organisms is attributed to the patient's normal endogenous microbial fecal flora. The wound invasion of E. coli is clearly due to poor hospital hygiene [44].
c. Pseudomonas Aeruginosa:
SSI can be due to Pseudomonas Aeruginosa in multiple surgical procedures [40]. These aerobic Gram-negative bacteria are usually found in moist environments and can cause opportunistic infections in the healthcare setting [45]. Moreover, a P. aeruginosa wound infection can persist for weeks and is highly resistant to antibiotic treatment, and underlying conditions such as diabetes can extend the persistence time of these infections thus exacerbating the infections [46].
d. Anaerobic Bacteria:
Anaerobic bacteria have also been implicated as causative agents in surgical infections. Gram-negative bacilli (mostly Bacteroides fragilis spp.), Gram-positive cocci, and Clostridium spp. were the top three anaerobes isolated from the cases. Furthermore, the most common presentations in these patients were deep-seated abscesses, infected non-healing ulcers, diabetic foot infections, and gas gangrene [47].
4. Biomarkers
a. CRP & PCT:
C-reactive protein (CRP) is a plasma-secreted acute-phase reactant that is primarily produced by the liver, along with lymphocytes and macrophages, in response to inflammation, infection, tissue injury, and cancer [48]. Pro-inflammatory cytokines, primarily IL-6 and, to a lesser extent, tumor necrosis factor-alpha (TNF-) and IL-1, stimulate its synthesis [49]. In case of infection, CRP promotes bacterial phagocytosis by binding to bacterial polysaccharides, aiding in neutrophils and macrophages opsonization, and activating the conventional complement system. CRP levels continue to rise 6 hours after the initial stimulation, reaching a peak in 48 hours, and after the trigger for inflammation is removed, CRP is catabolized by hepatocytes and swiftly cleared from circulation [48].
A useful diagnostic tool for identifying and keeping track of postoperative wound infections is C-reactive protein [50]. The increase in CRP level on the third postoperative day, however, is suggestive of wound infection, so keeping an eye on C-reactive protein levels can aid in designing an effective intervention, identifying problems earlier, and improving the prognosis for patients with postoperative infections [51].
Furthermore, (Petel et al., 2018) have indicated that CRP-driven antibiotic therapy was related to a decrease in the duration of antibiotic usage in neonatal patients and a decrease in the beginning of antibiotics in adult outpatients [48].
Another trustworthy biomarker for the diagnosis of SSIs is procalcitonin (PCT), particularly in the first few days following surgery [52]. The C-cells in the thyroid and, to a lesser extent, other neuroendocrine cells produce PCT, the precursor to the hormone calcitonin. In response to bacterial infection, production of PCT is triggered in all parenchymal tissues through the cytokines IL-6, TNF-α, and IL1- β. It is considered a more specific marker for bacterial infections because interferon-γ, which is typically secreted in response to viral infection, inhibits PCT production. When a bacterial infection is present, PCT rises; the magnitude of the rise correlates with the severity of the infection, and the concentration declines often indicating the remission of the sickness [53]. Following the release of TNF-and IL1- . It is considered a more specific marker for bacterial infections because interferon-γ, which is typically secreted in response to viral infection, inhibits PCT production. When a bacterial infection is present, PCT rises; the magnitude of the rise correlates with the severity of the infection, and the concentration declines often indicating the remission of the sickness [53]. Following the release of TNF-at 90 minutes and IL-6 at 3 hours following an infection, PCT is measurable 3 to 4 hours later. Its half-life is roughly 24 hours, and it peaks between 6 and 12 hours [53,54]. It is helpful for diagnosis, disease progression monitoring, and antibiotic therapy recommendations due to its favorable kinetic profile, specificity, and sensitivity to bacterial infection [53]. As a result of the faster increase and earlier peak at 24 h following infection, as well as the faster fall following infection resolution, PCT offers greater advantages than CRP [53,55].
These biomarkers serve as reliable indicators for SSI diagnostic prediction. However, they cannot be relied upon to accurately predict surgical site infections, so the diagnosis of SSI should be made in conjunction with the patient's clinical symptoms [52].
b. White Blood Cell Count (WBC) and Differential:
WBC maturation and release into the bloodstream are controlled by a number of variables, including interleukins, colony-stimulating factors, tumor necrosis factors, and complement components. Neutrophils are increasingly discharged into the bloodstream in response to infection or inflammation, where they remain for just a few hours while receiving additional supply from the bone marrow. The bone marrow increases the production and release of both band cells and immature neutrophil cells into circulation when the bone marrow's neutrophil reserve becomes depleted, a phenomenon known as the left shift [56].
From the onset of infection till recovery, there are multiple phases that characterize the change in WBC count and left shift which can be used to direct antibiotic therapy [57]. Neutrophils move into the infected location during the very first phase of infection, which accounts for the drop in WBC count in the blood [56]. The bone marrow begins manufacturing and releasing band cells into circulation (left-shift) a few hours later when the neutrophil pool in the bone marrow runs out and the WBC count drops below the normal level [56,57]. Thus, the WBC count begins to rise with persistent left shift within 20 hours of infection [57]. The high WBC count remains after initiating treatment, but band counts start to fall, indicating that the bone marrow no longer has to produce as many neutrophils. Finally, the WBC count returns to normal range without a left shift after bacterial infection is fully treated [56,57].
As a result, it is challenging to forecast bacterial infection using just one of these factors because they fluctuate interchangeably throughout the infection, and not all severe bacterial illnesses exhibit the same alterations in WBC and band count, while viral infections and trauma exhibit a left shift and a low WBC level [57].
A decrease in lymphocyte count accompanied by an increase in CRP and an increase in Neutrophil-Lymphocyte Ratio (NLR) signals that these parameters could be used as a reliable screening marker for SSIs 4 days after surgery, which is important for early diagnosis and infection control [58]. Thus, it is possible to employ WBCs and their subpopulations as crucial indicators of surgical site infections [59].
AGEs, angiotensin II, oxidative stress, and cytokines induce the WBC in diabetic patients to release cytokines and transcription factors which aid the disease's inflammatory process [60]. WBCs also produce proteases and superoxide molecules, which tend to increase oxidative stress [61]. Therefore, a greater WBC count, even when it falls within the normal range, is a sign of chronic inflammation and macro and microvascular complications seen in type 2 diabetic patients [60]. Along with the inflammatory processes, diabetic patients are more prone to tissue damage and have greater vulnerability to invasive microorganisms since the alteration in neutrophil activity renders them more susceptible to infections [61].
c. Glycemic Biomarkers:
Through several mechanisms, hyperglycemia results in endothelial dysfunction, which is at the origin of both the micro- and macrovascular complications in diabetes, including neuropathy, retinopathy, and nephropathy, as well as cardiovascular diseases, which can eventually lead to multiorgan failure. These mechanisms include increased oxidative stress, increased production of advanced glycation end-products, increased production of growth factors, cytokines, and angiotensin II, as well as enhanced polyol and hexosamine pathways, and diacylglycerol (DAG)-protein kinase C (PKC) [62].
The main parameter used to evaluate long-term glycemic control is hemoglobin A1c (HbA1c) since it reflects the average plasma glucose over the preceding months [63]. Improved glycemic control has been linked to a lower risk of developing and worsening micro- and macrovascular problems [64]. However, there is no proven HbA1c threshold below which complications are fully reduced, therefore achieving as close to normal glycemic control as feasible is the goal. HbA1c assessment, on the other hand, provides little about individual daily glucose changes, which are primarily measured by fasting plasma glucose (FPG) and postprandial glucose (PPG) levels. Therefore, in order to control the problems of both acute and chronic hyperglycemia, therapeutic decisions should be based on the three factors [62].
d. Interleukins and Cytokines:
The body's response to injury or infection is primarily dependent on the release of cytokines. The innate immune cells are signaled to invade the site of injury and infection by the release of cytokines, which in turn trigger the proper inflammatory or adaptive response. Epithelial and endothelial cells are among the first cells to begin this process. TNF, IL6, and IL1 are a few of the main pro-inflammatory cytokines and chemokines that the innate immune cells release [65].
Increased secretion of pro-inflammatory cytokines (TNF and IL6) and diminished production of anti-inflammatory mediators (IL4 and IL10) was linked to insulin resistance [66]. TNF and IL6 disrupt hepatic insulin signaling by reducing the liver's glucose absorption and glycogen signaling, respectively [67]. As a result, practically every aspect of immunity, inflammation, and the onset of diabetes is influenced by cytokines [66].
The serum marker IL-6 can be used to identify both early and delayed SSI [14]. It is a key factor in the development of fever and is in charge of promoting the production of acute-phase proteins, including CRP, and neutrophils in the bone marrow [68]. Because of this, the blood level of IL-6 reaches its peak and returns to normal faster than CRP, making IL-6 an effective inflammatory marker for early detection of bacterial infections. At a very early stage of the illness, serum IL-6 can distinguish between infection and an aseptic course with a very high degree of diagnostic accuracy. Thus, in order to enable early detection and treatment, the surgeon should concentrate primarily on SSI if IL-6 values are above 15.3 pg/ml [69].
e. Matrix Metalloproteinases (MMPs):
The matrix metalloproteinase (MMPs) family of endopeptidases is essentially engaged in several stages of wound healing, aiding in rapid wound recovery. They are dependent on zinc, hidden inside the extracellular matrix, and responsible for eliminating all damaged proteins while being constantly stimulated by macrophages and keratinocytes. They are also responsible for secreting chemokines, cytokines, lipid mediators, and antimicrobial peptides that are involved in the inflammatory stage of wound healing [70].
MMPs are subject to profound regulatory mechanisms that sustain homeostasis both at the intracellular and extracellular levels because of their great proteolytic ability [71]. High serum MMP concentrations were found in both kinds of diabetes, owing to the fact that hyperglycemia increases MPP expression and activity via oxidative stress or AGEs, which impairs wound remodeling capacity [72].
The inflammatory response during surgery causes the wound to become non-healing due to a change in the balance between extracellular matrix deposition and breakdown [71,72]. In order to evaluate the biomarkers that reflect wound status, (Saleh et al.,2019) utilized wound fluid. They came to the conclusion that MMP levels were higher in inflamed wounds, which resulted in excessive extracellular matrix breakdown and poor healing. MMPs can therefore be utilized as a biomarker that indicates the state of a wound; however, it is still being determined at what point biomarkers are needed to forecast the onset of SSIs [73].
f. Microbiome Biomarkers:
The skin microbiome is made up of a collection of microorganisms that live on the body's surface. As it secures the epithelium, controls the immune response, and shields it from infections, it aids in the skin barrier function [74].
On the other hand, the wound microbiome may have two distinct effects. First, by avoiding and treating bacterial wound infections and promoting quick wound closure, commensal skin bacteria can support a healthy repair process [75]. With the help of toll-like receptor-2 signaling and the production of lipoteichoic acid, bacteria like Staphylococcus epidermidis reduce inflammation following skin damage [76,77]. By creating antimicrobial compounds and proteases, as well as reducing the production of biofilm, it limits the colonization and invasion of S. aureus [75]. Additionally, Staphylococcus capitis, another bacterium, defends the host by reducing S. aureus' pathogenicity [74].
When inflammation remains as a result of the wound failing to progress through the stages of normal wound repair, it creates an environment for bacterial infiltration and multiplication, leading to a chronic wound infection, which is a critical component in delayed wound healing [75].
Near the incision, during surgery, skin-invading microbes that are a part of the skin microbiome can result in SSIs [78]. Additionally, because bacteria are not evenly distributed throughout the body, the local microbiome close to the incision site can serve as a marker for locating the source of SSIs [79]. P. Acnes is more common on the upper back and chest, which have disproportionately more sebaceous glands than other areas. (Wenzel et al., 2019) has revealed that infections following shoulder surgery, where sebaceous glands are more common, would exhibit an overrepresentation of this organism more frequently than infections following knee or hip surgery. Therefore, this bacterium serves as a flag for SSIs in this specific site. This highlights the significance of managing the microbiome at the surgical site, which would undoubtedly reduce the risks of SSIs [78].
5. Complications
Complications from SSI can be local or systemic, with local complications including delayed healing, cellulitis, and abscesses and systemic complications including sepsis and organ failure [5].
a. Prolonged hospitalization and increased cost:
A major consequence of SSI in diabetics is delayed wound healing due to prolonged SSI time [80]. As a result, these patients, particularly those with complex diabetes, have a longer duration of infection and require more insulin [26]. According to (Neumayer et al, 2007), the average increase in hospital stay following surgery in patients with SSI was roughly 4 days [81]. This rise is caused not only by delayed healing, but also by diabetes, which raises the chance of infection severity, requiring hospitalization and, in some cases, amputation [82]. This extended stay will raise costs for both the medical facility and the patient [83]. According to (Jenks et al, 2014) who looked at the impact of SSI following vascular surgery, the median additional postoperative length of stay linked with SSI was 10 days, with an average cost of £2480 per episode [84]. Other side effects of extended hospitalization include pressure-related harm to the skin, venous thromboembolism, deconditioning and frailty, and, eventually, an increase in mortality [85].
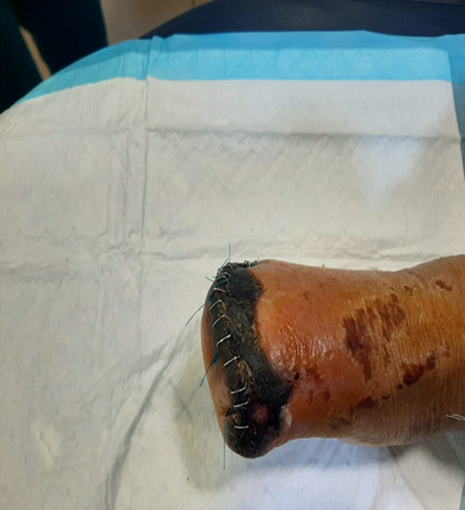
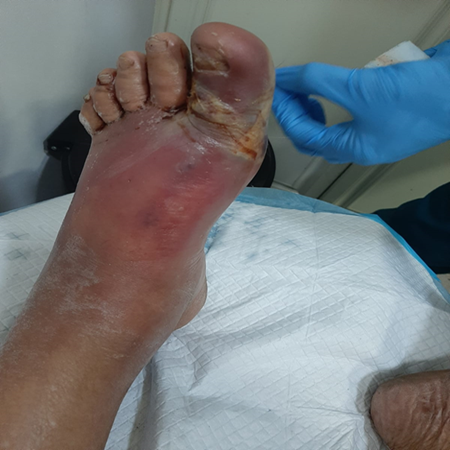
b. Local complications and Necrotizing Fasciitis:
Local SSI consequences include delayed wound healing, abscesses, and cellulitis [5]. Diabetes exacerbates this illness because it impairs the immune system, induces neuropathy, and reduces blood supply to the infection site [86]. Skin and soft-tissue infections (SSTI) problems are five times more common in diabetes patients, necessitating greater hospitalization [87]. The most serious of these consequences include abscesses, cellulitis, and NF [86]. (Suaya et al, 2013) found that diabetes patients had a considerably greater incidence of abscess [87]. Alcohol abuse, cancer, chronic cardiac and renal illness, intravenous drug use, immunosuppressive medication, and immunosuppressive malnutrition all enhance the risk of NF [88]. Diabetes has a significant impact on the immune system and is a common contributing medical condition for NF and limb loss [89].
c. Sepsis and organ dysfunction:
Sepsis is described as an abnormal immunological response to an infection that results in organ dysfunction [90]. Sepsis can arise during surgery and is worsened by incisional infection [91]. According to (Barie et al.,2018) in the United States, about two million surgeries are complicated by SSI each year, and surgical patients account for 30% of patients with sepsis [92]. Diabetic individuals tend to have a greater risk of sepsis and septicemia than non-diabetic patients [93]. Diabetes causes significant metabolic changes in the body, including increased AGE formation, stimulation of protein kinase C isoforms, and raised flux through the polyol and hexosamine pathways, which leads to increased superoxide production and activation of inflammatory pathways in diabetic patients [90]. Furthermore, the immune system is being weakened by the effect of excessive glucose on immune cells, cytokines, and immunoglobins. All of these effects enhance the likelihood of sepsis in these individuals [86,93]. In the absence of organ failure, sepsis is not deemed present [90,92]. According to (Barie et al, 2008), sepsis is responsible for up to three-quarters of instances of multiple organ failure syndrome in postoperative patients [94]. Septic individuals are at an increased risk of having acute renal failure, cardiovascular failure, and other dysfunctions. Diabetic individuals with sepsis, on the other hand, appear to be at a greater risk of developing acute renal failure [95].
d. Psychosocial:
Patients with diabetic foot infection, regardless of the source, have a worse quality of life, are more likely to suffer from depression, have a lower level of education, and are less self-sufficient. This is exacerbated by stress and unemployment following amputation [96]. Furthermore, individuals with SSI have a low quality of life because they struggle to engage with others, resulting in strained relationships with family and friends, lost income, and financial hardship. Some of these individuals also mention a dysfunctional doctor-patient interaction [97]. Physical handicaps accompanied by depressive symptoms as a result of SSI have an influence on the patient's life throughout their hospital stay and beyond discharge, with some patients showing signs of depression even 6 months after departure [85]. However, SSIs delay but do not prevent long-term increases in quality of life, implying that SSIs have no effect on long-term quality of life [98]. These negative SSI implications will necessitate increasing financial expenses to handle [85].
Table 1: This table lists the most common types of etiological factors and their complications in diabetic patients.
| Infections | Complications |
| Staphylococcus aureus |
|
| E. coli | |
| Klebsiella species | |
| Enterococcus species |
6. Preoperative, intraoperative, and postoperative management
Perioperative hyperglycemia, as previously stated, increases the risk of surgical complications including poor wound healing and surgical site [99,100]. Thus, blood glucose should be monitored and controlled to minimize the risk of these complications [100]. For this reason, the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have proposed 140 to 180 mg per dl as a glycemic target [101]. In addition to that, Hb1Ac is recommended to be 7-8%. There is no HbA1c threshold above which surgery should be postponed because Hb1Ac alone is insufficient to predict the likelihood of postoperative infections [102,103]. Moreover, guidelines suggest close and consistent monitoring of glucose electronically to enable early intervention in case of dysglycemia [104].
It Is important to mention that oral hypoglycemics and insulin taken by diabetics should be adjusted during the perioperative period. For instance, most medications including metformin are withheld on the day of surgery. Sodium glucose-linked transporter-2 (SGLT-2) inhibitors specifically, should be avoided 3-4 days before surgery considering the risk of developing diabetic ketoacidosis [102,104,105]. In T1D patients, SC basal insulin should never be interrupted [106] and long-acting or premixed insulin can be taken until the day of surgery with a reduction of 25% of the dose. If hypoglycemia occurs, insulin should be withheld [102,104]. Intraoperatively, hyperglycemia (>180 mg/dl) is treated with insulin infusion in critically ill patients who have hemodynamic changes, significant blood loss, requirement for inotropes and vasopressors, and long surgeries. However, continuous SC insulin is recommended in non-critically ill patients, with no long postoperative fasting period, and in short operative procedures (less than 4 hours) [102].
Furthermore, in perioperative care, nausea and vomiting need to be minimized. According to a meta-analysis conducted in 2020, several antiemetics medicines (aprepitant, ramosetron, granisetron, dexamethasone, and ondansetron) have been proven to be effective [107]. Several concerns have been expressed about dexamethasone because of its influence on hyperglycemia, particularly at doses of more than 8 mg IV. For this reason, a dose of 4-5 mg in well-controlled diabetes patients is suggested [108,109]. Despite this, current evidence shows that dexamethasone has no higher risk of SSI as compared to placebo. Further investigations are needed to inform practice about the dose effect of dexamethasone on glycemia [110].
Regarding antibiotic prophylaxis, limited studies address the use of antibiotic prophylaxis in diabetic patients [111]. From the available data, we can deduce that antibiotic prophylaxis has no effect on reducing SSIs in diabetic patients in the context of hand surgeries [111,112]. (Ko et al., 2017) found that antibiotic prophylaxis has no significant benefit in well-controlled diabetes individuals over nondiabetic patients. Whereas in poorly controlled patients (Hb1Ac >7), the immune system due to glucose will be altered, and thus antibiotic prophylaxis benefits diabetic patients [113]. Furthermore, (Yao et al., 2022) revealed that diabetes does not require long-term antibiotic prophylaxis in gastric cancer surgery [114]. Also, in common periodontal operations, no scientific evidence demonstrates the effectiveness of antibiotics prophylaxis as a precautionary measure [115]. Guidelines for patients having surgery, including those with diabetes, recommend using cefazolin, a narrow-spectrum antibiotic, for most surgical operations and cefoxitin for abdominal surgery. Antibiotic combinations (oral + intravenous (IV)) have been demonstrated to be more effective than either oral or IV antibiotics alone. In terms of administration time antibiotics should be given 60 minutes before incision [116]. Other precautions include maintaining normal body temperature, removing hair with clippers, combining the antiseptic chlorhexidine gluconate with an alcohol-based preparation, and employing negative pressure wound care [117]. In addition to that, treating sutures with triclosan, an antimicrobial agent, reduces SSI. These sutures act as a reservoir that releases its agents over time and prevents infection from being established and spread. According to a recent meta-analysis, triclosan-coated sutures reduce SSIs [118]. Finally, continuous transdermal and subcutaneous sutures are preferred for better wound healing and thus reduce surgical infections [119].
In short, glucose monitoring in pre-, intra-, and postoperative periods, is the mainstream strategy to reduce SSIs in diabetic patients along with other standard procedures.
Conclusion
Diabetes is a chronic condition marked by hyperglycemia that, if unregulated, can be fatal. Diabetic people who have surgery are at a considerable risk of acquiring SSI thereafter. Diabetes delays wound healing and renders patients more vulnerable to SSI due to factors including hyperglycemia and neuropathy and exacerbates consequences like deep tissue infections and sepsis. Patients with diabetes undergoing surgery must have an early diagnosis using biomarkers and strict management to avoid complications such as sepsis, necrotizing fasciitis, and poor quality of life. Healthcare providers should make an effort to decrease the considerable healthcare burden imposed by these disorders by addressing diabetes management, implementing infection prevention measures, and providing patient-centered care, ultimately improving diabetes outcomes.
Legend
- Diabetes Mellitus: DM
- Type 1 diabetes mellitus: T1DM
- Type 2 diabetes mellitus: T1DM
- Surgical site infections: SSI
- Escherichia coli: E. coli
- Protein kinase C: PKC
- Advanced glycation end products: AGEs
- Interleukin: IL
- Staphylococcus aureus: s. aureus
- P. Aeruginosa: Pseudomonas aeruginosa
- Methicillin-resistant Staphylococcus aureus: MRSA
- C-reactive protein: CRP
- Procalcitonin: PCT
- Tumor necrosis factor-alpha: TNFa
- White blood cells: WBC
- Matrix metalloproteinases: MMPs
- haemoglobin A1c: HbA1c
- Skin and soft tissue infections: SSTI
- Necrotizing fasciitis: NF
- American Diabetes Association: ADA
- American Association of Clinical Endocrinologists: AACE
- Sodium and glucose linked transporter 2: SGLT-2
- Intravenous: IV
Declarations
Funding: Not applicable
Conflicts of interest: The authors declare that they have no conflict of interest.
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and material (data transparency): All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Code availability: Not applicable
Acknowledgment: The authors thank Dr. Hiba Hamdar (hiba.hamdar@live.com), who supervised us reviewed our article, and gave us her valuable comments.
Authors' contributions: Each author has contributed in the same manner to this manuscript
References
- Kharroubi, A. T., & Darwish, H. M. (2015). Diabetes mellitus: The epidemic of the century. World journal of diabetes, 6(6), 850.
View at Publisher | View at Google Scholar - Sapra A, Bhandari P. Diabetes. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 21, 2023.
View at Publisher | View at Google Scholar - Rush, T., McGeary, M., Sicignano, N., & Buryk, M. A. (2018). A plateau in new onset type 1 diabetes: Incidence of pediatric diabetes in the United States Military Health System. Pediatric diabetes, 19(5), 917-922.
View at Publisher | View at Google Scholar - Martin, E. T., Kaye, K. S., Knott, C., Nguyen, H., Santarossa, M., Evans, R., ... & Jaber, L. (2016). Diabetes and risk of surgical site infection: a systematic review and meta-analysis. infection control & hospital epidemiology, 37(1), 88-99.
View at Publisher | View at Google Scholar - Zabaglo M, Sharman T. Postoperative Wound Infection. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 3, 2023.
View at Publisher | View at Google Scholar - Young, P. Y., & Khadaroo, R. G. (2014). Surgical site infections. Surgical Clinics, 94(6), 1245-1264.
View at Publisher | View at Google Scholar - Ansari, S., Hassan, M., Barry, H. D., Bhatti, T. A., Hussain, S. Z. M., Jabeen, S., & Fareed, S. (2019). Risk factors associated with surgical site infections: a retrospective report from a developing country. Cureus, 11(6).
View at Publisher | View at Google Scholar - Olsen, M. A., Nepple, J. J., Riew, K. D., Lenke, L. G., Bridwell, K. H., Mayfield, J., & Fraser, V. J. (2008). Risk factors for surgical site infection following orthopaedic spinal operations. JBJS, 90(1), 62-69.
View at Publisher | View at Google Scholar - Liu, J. M., Deng, H. L., Chen, X. Y., Zhou, Y., Yang, D., Duan, M. S., ... & Liu, Z. L. (2018). Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine, 43(10), 732-737.
View at Publisher | View at Google Scholar - Alkaaki, A., Al-Radi, O. O., Khoja, A., Alnawawi, A., Alnawawi, A., Maghrabi, A., ... & Aljiffry, M. (2019). Surgical site infection following abdominal surgery: a prospective cohort study. Canadian journal of surgery, 62(2), 111.
View at Publisher | View at Google Scholar - Ali, A., Gebretsadik, D., & Desta, K. (2023). Incidence of surgical site infection, bacterial isolate, and their antimicrobial susceptibility pattern among patients who underwent surgery at Dessie Comprehensive Specialized Hospital, Northeast Ethiopia. SAGE Open Medicine, 11, 20503121231172345.
View at Publisher | View at Google Scholar - Almadani, Y. H., Vorstenbosch, J., Davison, P. G., & Murphy, A. M. (2021, July). Wound healing: A comprehensive review. In Seminars in plastic surgery (Vol. 35, No. 03, pp. 141-144). 333 Seventh Avenue, 18th Floor, New York, NY 10001, USA: Thieme Medical Publishers, Inc..
View at Publisher | View at Google Scholar - Gonzalez, A. C. D. O., Costa, T. F., Andrade, Z. D. A., & Medrado, A. R. A. P. (2016). Wound healing-A literature review. Anais brasileiros de dermatologia, 91, 614-620.
View at Publisher | View at Google Scholar - Ozgok Kangal MK, Regan JP. Wound Healing. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 1, 2023.
View at Publisher | View at Google Scholar - Patel, S., Srivastava, S., Singh, M. R., & Singh, D. (2019). Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomedicine & Pharmacotherapy, 112, 108615.
View at Publisher | View at Google Scholar - Burgess, J. L., Wyant, W. A., Abdo Abujamra, B., Kirsner, R. S., & Jozic, I. (2021). Diabetic wound-healing science. Medicina, 57(10), 1072.
View at Publisher | View at Google Scholar - Berlanga-Acosta, J., Schultz, G. S., López-Mola, E., Guillen-Nieto, G., García-Siverio, M., & Herrera-Martínez, L. (2013). Glucose toxic effects on granulation tissue productive cells: the diabetics’ impaired healing. BioMed research international, 2013.
View at Publisher | View at Google Scholar - Deng, L., Du, C., Song, P., Chen, T., Rui, S., Armstrong, D. G., & Deng, W. (2021). The role of oxidative stress and antioxidants in diabetic wound healing. Oxidative medicine and cellular longevity, 2021.
View at Publisher | View at Google Scholar - Dworzański, J., Strycharz-Dudziak, M., Kliszczewska, E., Kiełczykowska, M., Dworzańska, A., Drop, B., & Polz-Dacewicz, M. (2020). Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. Plos one, 15(3), e0230374.
View at Publisher | View at Google Scholar - Barker, A. R., Rosson, G. D., & Dellon, A. L. (2006). Wound healing in denervated tissue. Annals of plastic surgery, 57(3), 339-342.
View at Publisher | View at Google Scholar - Mallik, S. B., Jayashree, B. S., & Shenoy, R. R. (2018). Epigenetic modulation of macrophage polarization-perspectives in diabetic wounds. Journal of Diabetes and its Complications, 32(5), 524-530.
View at Publisher | View at Google Scholar - Rehman, Z. U., Khan, J., & Noordin, S. (2023). Diabetic foot ulcers: Contemporary assessment and management. JPMA. The Journal of the Pakistan Medical Association, 73(7), 1480-1487.
View at Publisher | View at Google Scholar - Vallianou, N., Evangelopoulos, A., & Koutalas, P. (2009). Alpha-lipoic acid and diabetic neuropathy. The review of diabetic studies: RDS, 6(4), 230.
View at Publisher | View at Google Scholar - Volmer-Thole, M., & Lobmann, R. (2016). Neuropathy and diabetic foot syndrome. International journal of molecular sciences, 17(6), 917.
View at Publisher | View at Google Scholar - Weigelt, J. A. (2010). Diabetic foot infections: diagnosis and management. Surgical Infections, 11(3), 295-298.
View at Publisher | View at Google Scholar - Wukich, D. K., McMillen, R. L., Lowery, N. J., & Frykberg, R. G. (2011). Surgical site infections after foot and ankle surgery: a comparison of patients with and without diabetes. Diabetes Care, 34(10), 2211-2213.
View at Publisher | View at Google Scholar - Strain, W. D., & Paldánius, P. M. (2018). Diabetes, cardiovascular disease and the microcirculation. Cardiovascular diabetology, 17, 1-10.
View at Publisher | View at Google Scholar - Paneni, F., Beckman, J. A., Creager, M. A., & Cosentino, F. (2013). Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. European heart journal, 34(31), 2436-2443.
View at Publisher | View at Google Scholar - Madonna, R., Balistreri, C. R., Geng, Y. J., & De Caterina, R. (2017). Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascular Pharmacology, 90, 1-7.
View at Publisher | View at Google Scholar - Shilling, A. M., & Raphael, J. (2008). Diabetes, hyperglycemia, and infections. Best Practice & Research Clinical Anaesthesiology, 22(3), 519-535.
View at Publisher | View at Google Scholar - Spindler, M. P., Ho, A. M., Tridgell, D., McCulloch‐Olson, M., Gersuk, V., Ni, C., ... & Sanda, S. (2016). Acute hyperglycemia impairs IL‐6 expression in humans. Immunity, inflammation and disease, 4(1), 91-97.
View at Publisher | View at Google Scholar - Xia, C., Rao, X., & Zhong, J. (2017). Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. Journal of diabetes research, 2017.
View at Publisher | View at Google Scholar - DeFuria, J., Belkina, A. C., Jagannathan-Bogdan, M., Snyder-Cappione, J., Carr, J. D., Nersesova, Y. R., ... & Nikolajczyk, B. S. (2013). B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences, 110(13), 5133-5138.
View at Publisher | View at Google Scholar - Frühbeck, G., Busetto, L., Dicker, D., Yumuk, V., Goossens, G. H., Hebebrand, J., ... & Toplak, H. (2019). The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obesity facts, 12(2), 131-136.
View at Publisher | View at Google Scholar - Darlenski, R., Mihaylova, V., & Handjieva-Darlenska, T. (2022). The link between obesity and the skin. Frontiers in nutrition, 9, 855573.
View at Publisher | View at Google Scholar - Cotter, C., & Walsh, S. (2021). Cutaneous sequelae of a national health crisis: obesity and the skin. Skin Health and Disease, 1(1), e7.
View at Publisher | View at Google Scholar - S. Earlam A, Woods L. Obesity - American Nurse Journal. 2020. https://www.myamericannurse.com/wp-content/uploads/2020/04/Obesity-Skin.pdf.
View at Publisher | View at Google Scholar - Frasca, D., & Strbo, N. (2022). Effects of obesity on infections with emphasis on skin infections and wound healing. Journal of dermatology and skin science, 4(3), 5.
View at Publisher | View at Google Scholar - Kolasiński, W. (2019). Zakażenia miejsca operowanego–przegląd aktualnej wiedzy, metody zapobiegania. Polish Journal of Surgery, 91(4), 41-47.
View at Publisher | View at Google Scholar - Thurlow, L. R., Stephens, A. C., Hurley, K. E., & Richardson, A. R. (2020). Lack of nutritional immunity in diabetic skin infections promotes Staphylococcus aureus virulence. Science Advances, 6(46), eabc5569.
View at Publisher | View at Google Scholar - Anderson, M. J., David, M. L., Scholz, M., Bull, S. J., Morse, D., Hulse-Stevens, M., & Peterson, M. L. (2015). Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares. Antimicrobial agents and chemotherapy, 59(5), 2765-2773.
View at Publisher | View at Google Scholar - Dryden, M., Baguneid, M., Eckmann, C., Corman, S., Stephens, J., Solem, C., ... & Haider, S. (2015). Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clinical Microbiology and Infection, 21, S27-S32.
View at Publisher | View at Google Scholar - Negi, V., Pal, S., Juyal, D., Sharma, M. K., & Sharma, N. (2015). Bacteriological profile of surgical site infections and their antibiogram: A study from resource constrained rural setting of Uttarakhand state, India. Journal of clinical and diagnostic research: JCDR, 9(10), DC17.
View at Publisher | View at Google Scholar - Tosh, P. K., Disbot, M., Duffy, J. M., Boom, M. L., Heseltine, G., Srinivasan, A., ... & Berríos-Torres, S. I. (2011). Outbreak of Pseudomonas aeruginosa surgical site infections after arthroscopic procedures: Texas, 2009. Infection Control & Hospital Epidemiology, 32(12), 1179-1186.
View at Publisher | View at Google Scholar - Turner, K. H., Everett, J., Trivedi, U., Rumbaugh, K. P., & Whiteley, M. (2014). Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS genetics, 10(7), e1004518.
View at Publisher | View at Google Scholar - Ananth-Shenoy, P., Vishwanath, S., Targain, R., Shetty, S., Sunil-Rodrigues, G., Mukhopadhyay, C., & Chawla, K. (2016). Anaerobic infections in surgical wards: a two year study. Iranian journal of microbiology, 8(3), 181.
View at Publisher | View at Google Scholar - Petel, D., Winters, N., Gore, G. C., Papenburg, J., Beltempo, M., Lacroix, J., & Fontela, P. S. (2018). Use of C-reactive protein to tailor antibiotic use: a systematic review and meta-analysis. Bmj Open, 8(12), e022133.
View at Publisher | View at Google Scholar - Sproston, N. R., & Ashworth, J. J. (2018). Role of C-reactive protein at sites of inflammation and infection. Frontiers in immunology, 9, 754.
View at Publisher | View at Google Scholar - Chapman, G., Holton, J., & Chapman, A. (2016). A threshold for concern? C-reactive protein levels following operatively managed neck of femur fractures can detect infectious complications with a simple formula. Clinical biochemistry, 49(3), 219-224.
View at Publisher | View at Google Scholar - Shetty, S., Ethiraj, P., & Shanthappa, A. H. (2022). C-Reactive protein is a diagnostic tool for postoperative infection in orthopaedics. Cureus, 14(2).
View at Publisher | View at Google Scholar - Zheng, S., Wang, Z., Qin, S., & Chen, J. T. (2020). Usefulness of inflammatory markers and clinical manifestation for an earlier method to diagnosis surgical site infection after spinal surgery. International orthopaedics, 44, 2211-2219.
View at Publisher | View at Google Scholar - Samsudin, I., & Vasikaran, S. D. (2017). Clinical utility and measurement of procalcitonin. The Clinical Biochemist Reviews, 38(2), 59.
View at Publisher | View at Google Scholar - Becker, K. L., Snider, R., & Nylen, E. S. (2010). Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. British journal of pharmacology, 159(2), 253-264.
View at Publisher | View at Google Scholar - Nargis, W., Ibrahim, M. D., & Ahamed, B. U. (2014). Procalcitonin versus C-reactive protein: Usefulness as biomarker of sepsis in ICU patient. International journal of critical illness and injury science, 4(3), 195.
View at Publisher | View at Google Scholar - Honda, T., Uehara, T., Matsumoto, G., Arai, S., & Sugano, M. (2016). Neutrophil left shift and white blood cell count as markers of bacterial infection. Clinica Chimica Acta, 457, 46-53.
View at Publisher | View at Google Scholar - Ishimine, N., Honda, T., Yoshizawa, A., Kawasaki, K., Sugano, M., Kobayashi, Y., & Matsumoto, T. (2013). Combination of White Blood Cell Count and Left Shift Level Real‐Timely Reflects a Course of Bacterial Infection. Journal of clinical laboratory analysis, 27(5), 407-411.
View at Publisher | View at Google Scholar - Shen, C. J., Miao, T., Wang, Z. F., Li, Z. F., Huang, L. Q., Chen, T. T., & Yan, W. H. (2019). Predictive value of post-operative neutrophil/lymphocyte count ratio for surgical site infection in patients following posterior lumbar spinal surgery. International immunopharmacology, 74, 105705.
View at Publisher | View at Google Scholar - Iwata, E., Shigematsu, H., Koizumi, M., Nakajima, H., Okuda, A., Morimoto, Y., ... & Tanaka, Y. (2016). Lymphocyte count at 4 days postoperatively and CRP level at 7 days postoperatively. Spine, 41(14), 1173-1178.
View at Publisher | View at Google Scholar - Chung, F. M., Tsai, J. C. R., Chang, D. M., Shin, S. J., & Lee, Y. J. (2005). Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes care, 28(7), 1710-1717.
View at Publisher | View at Google Scholar - Adane, T., Asrie, F., Getaneh, Z., & Getawa, S. (2021). White blood cells and platelet profiles of diabetic patients at University of Gondar specialized referral hospital: A comparative cross‐sectional study. Journal of clinical laboratory Analysis, 35(6), e23808.
View at Publisher | View at Google Scholar - Marcovecchio, M. L., Lucantoni, M., & Chiarelli, F. (2011). Role of chronic and acute hyperglycemia in the development of diabetes complications. Diabetes technology & therapeutics, 13(3), 389-394.
View at Publisher | View at Google Scholar - Monnier, L., Colette, C., & Owens, D. (2011). Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes technology & therapeutics, 13(S1), S-25.
View at Publisher | View at Google Scholar - Rakhis Sr, S. A. B., AlDuwayhis, N. M., Aleid, N., AlBarrak, A. N., & Aloraini, A. A. (2022). Glycemic control for type 2 diabetes mellitus patients: A systematic review. Cureus, 14(6).
View at Publisher | View at Google Scholar - Rakhis Sr, S. A. B., AlDuwayhis, N. M., Aleid, N., AlBarrak, A. N., & Aloraini, A. A. (2022). Glycemic control for type 2 diabetes mellitus patients: A systematic review. Cureus, 14(6).
View at Publisher | View at Google Scholar - Stanley, A. C., & Lacy, P. (2010). Pathways for cytokine secretion. Physiology, 25(4), 218-229.
View at Publisher | View at Google Scholar - De Luca, C., & Olefsky, J. M. (2008). Inflammation and insulin resistance. FEBS letters, 582(1), 97-105.
View at Publisher | View at Google Scholar - Xiao, J., Li, J., Cai, L., Chakrabarti, S., & Li, X. (2014). Cytokines and diabetes research. Journal of diabetes research, 2014.
View at Publisher | View at Google Scholar - Fraunberger, P., Wang, Y., Holler, E., Parhofer, K. G., Nagel, D., Walli, A. K., & Seidel, D. (2006). Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock, 26(1), 10-12.
View at Publisher | View at Google Scholar - Lenski, M., Tonn, J. C., & Siller, S. (2021). Interleukin-6 as inflammatory marker of surgical site infection following spinal surgery. Acta Neurochirurgica, 163, 1583-1592.
View at Publisher | View at Google Scholar - Kandhwal, M., Behl, T., Singh, S., Sharma, N., Arora, S., Bhatia, S., ... & Bungau, S. (2022). Role of matrix metalloproteinase in wound healing. American Journal of Translational Research, 14(7), 4391.
View at Publisher | View at Google Scholar - Abreu, B. J., & de Brito Vieira, W. H. (2016). Metalloproteinase changes in diabetes. Metabolic Influences on Risk for Tendon Disorders, 185-190.
View at Publisher | View at Google Scholar - Ayuk, S. M., Abrahamse, H., & Houreld, N. N. (2016). The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. Journal of diabetes research, 2016.
View at Publisher | View at Google Scholar - Saleh, K., Strömdahl, A. C., Riesbeck, K., & Schmidtchen, A. (2019). Inflammation biomarkers and correlation to wound status after full-thickness skin grafting. Frontiers in medicine, 6, 159.
View at Publisher | View at Google Scholar - Harris-Tryon, T. A., & Grice, E. A. (2022). Microbiota and maintenance of skin barrier function. Science, 376(6596), 940-945.
View at Publisher | View at Google Scholar - Canchy, L., Kerob, D., Demessant, A. L., & Amici, J. M. (2023). Wound healing and microbiome, an unexpected relationship. Journal of the European Academy of Dermatology and Venereology, 37, 7-15.
View at Publisher | View at Google Scholar - Maheswary, T., Nurul, A. A., & Fauzi, M. B. (2021). The insights of microbes’ roles in wound healing: A comprehensive review. Pharmaceutics, 13(7), 981.
View at Publisher | View at Google Scholar - Li, D., Wang, W., Wu, Y., Ma, X., Zhou, W., & Lai, Y. (2019). Lipopeptide 78 from Staphylococcus epidermidis activates β-catenin to inhibit skin inflammation. The Journal of Immunology, 202(4), 1219-1228.
View at Publisher | View at Google Scholar - Wenzel, R. P. (2019). Surgical site infections and the microbiome: An updated perspective. Infection Control & Hospital Epidemiology, 40(5), 590-596.
View at Publisher | View at Google Scholar - Shiono, Y., Ishii, K., Nagai, S., Kakinuma, H., Sasaki, A., Funao, H., ... & Matsumoto, M. (2016). Delayed Propionibacterium acnes surgical site infections occur only in the presence of an implant. Scientific reports, 6(1), 32758.
View at Publisher | View at Google Scholar - Cheuk, N., Worth, L. J., Tatoulis, J., Skillington, P., Kyi, M., & Fourlanos, S. (2021). The relationship between diabetes and surgical site infection following coronary artery bypass graft surgery in current-era models of care. Journal of Hospital Infection, 116, 47-52.
View at Publisher | View at Google Scholar - Neumayer, L., Hosokawa, P., Itani, K., El-Tamer, M., Henderson, W. G., & Khuri, S. F. (2007). Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. Journal of the American College of Surgeons, 204(6), 1178-1187.
View at Publisher | View at Google Scholar - Wukich, D. K., Lowery, N. J., McMillen, R. L., & Frykberg, R. G. (2010). Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. JBJS, 92(2), 287-295.
View at Publisher | View at Google Scholar - Turner, M. C., & Migaly, J. (2019). Surgical site infection: the clinical and economic impact. Clinics in Colon and Rectal Surgery, 32(03), 157-165.
View at Publisher | View at Google Scholar - Jenks, P. J., Laurent, M., McQuarry, S., & Watkins, R. (2014). Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. Journal of Hospital infection, 86(1), 24-33.
View at Publisher | View at Google Scholar - Totty, J. P., Moss, J. W. E., Barker, E., Mealing, S. J., Posnett, J. W., Chetter, I. C., & Smith, G. E. (2021). The impact of surgical site infection on hospitalisation, treatment costs, and health‐related quality of life after vascular surgery. International Wound Journal, 18(3), 261-268.
View at Publisher | View at Google Scholar - Zhou, K., & Lansang, M. C. (2021). Diabetes mellitus and infections.
View at Publisher | View at Google Scholar - Suaya, J. A., Eisenberg, D. F., Fang, C., & Miller, L. G. (2013). Skin and soft tissue infections and associated complications among commercially insured patients aged 0–64 years with and without diabetes in the US. PLoS One, 8(4), e60057.
View at Publisher | View at Google Scholar - Goh, T., Goh, L. G., Ang, C. H., & Wong, C. H. (2014). Early diagnosis of necrotizing fasciitis. Journal of British Surgery, 101(1), e119-e125.
View at Publisher | View at Google Scholar - Cheng, N. C., Tai, H. C., Chang, S. C., Chang, C. H., & Lai, H. S. (2015). Necrotizing fasciitis in patients with diabetes mellitus: clinical characteristics and risk factors for mortality. BMC infectious diseases, 15, 1-9.
View at Publisher | View at Google Scholar - Frydrych, L. M., Fattahi, F., He, K., Ward, P. A., & Delano, M. J. (2017). Diabetes and sepsis: risk, recurrence, and ruination. Frontiers in endocrinology, 8, 271.
View at Publisher | View at Google Scholar - Malik, A. T., Jain, N., Scharschmidt, T. J., Mayerson, J. L., & Khan, S. N. (2018). Factors associated with post-operative sepsis following surgery for spinal tumors: An analysis of the ACS-NSQIP database. Clinical Neurology and Neurosurgery, 172, 1-7.
View at Publisher | View at Google Scholar - Barie, P. S. (2018). Outcomes of surgical sepsis. Surgical Infections, 19(2), 230-235.
View at Publisher | View at Google Scholar - Lin, C. S., Chang, C. C., Lee, Y. W., Liu, C. C., Yeh, C. C., Chang, Y. C., ... & Liao, C. C. (2019). Adverse outcomes after major surgeries in patients with diabetes: a multicenter matched study. Journal of Clinical Medicine, 8(1), 100.
View at Publisher | View at Google Scholar - Barie, P. S., Hydo, L. J., Pieracci, F. M., Shou, J., & Eachempati, S. R. (2009). Multiple organ dysfunction syndrome in critical surgical illness. Surgical infections, 10(5), 369-377.
View at Publisher | View at Google Scholar - Esper, A. M., Moss, M., & Martin, G. S. (2009). The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Critical care, 13, 1-6.
View at Publisher | View at Google Scholar - Fejfarová, V., Jirkovská, A., Dragomirecká, E., Game, F., Bém, R., Dubský, M., ... & Wu, S. (2014). Does the diabetic foot have a significant impact on selected psychological or social characteristics of patients with diabetes mellitus?. Journal of Diabetes Research, 2014.
View at Publisher | View at Google Scholar - Avsar, P., Patton, D., Ousey, K., Blackburn, J., O'Connor, T., & Moore, Z. (2021). The Impact of Surgical Site Infection on Health-related Quality of Life: A Systematic Review. Wound management & prevention, 67(6), 10-19.
View at Publisher | View at Google Scholar - Pennington, Z., Sundar, S. J., Lubelski, D., Alvin, M. D., Benzel, E. C., & Mroz, T. E. (2019). Cost and quality of life outcome analysis of postoperative infections after posterior lumbar decompression and fusion. Journal of Clinical Neuroscience, 68, 105-110.
View at Publisher | View at Google Scholar - Stryker, L. S., Abdel, M. P., Morrey, M. E., Morrow, M. M., Kor, D. J., & Morrey, B. F. (2013). Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. JBJS, 95(9), 808-814.
View at Publisher | View at Google Scholar - Kwon, S., Thompson, R., Dellinger, P., Yanez, D., Farrohki, E., & Flum, D. (2013). Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Annals of surgery, 257(1), 8.
View at Publisher | View at Google Scholar - Todd, L. A., & Vigersky, R. A. (2021). Evaluating perioperative glycemic control of non-cardiac surgical patients with diabetes. Military medicine, 186(9-10), e867-e872.
View at Publisher | View at Google Scholar - Duggan, E., & Chen, Y. (2019). Glycemic management in the operating room: screening, monitoring, oral hypoglycemics, and insulin therapy. Current diabetes reports, 19, 1-13.
View at Publisher | View at Google Scholar - Hagedorn, J. M., Bendel, M. A., Hoelzer, B. C., Aiyer, R., & Caraway, D. (2023). Preoperative hemoglobin A1c and perioperative blood glucose in patients with diabetes mellitus undergoing spinal cord stimulation surgery: A literature review of surgical site infection risk. Pain Practice, 23(1), 83-93.
View at Publisher | View at Google Scholar - Grant, B., & Chowdhury, T. A. (2022). New guidance on the perioperative management of diabetes. Clinical Medicine, 22(1), 41.
View at Publisher | View at Google Scholar - American Diabetes Association. (2021). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes care, 44(Supplement_1), S15-S33.
View at Publisher | View at Google Scholar - Cheisson, G., Jacqueminet, S., Cosson, E., Ichai, C., Leguerrier, A. M., Nicolescu-Catargi, B., ... & Benhamou, D. (2018). Perioperative management of adult diabetic patients. Preoperative period. Anaesthesia Critical Care & Pain Medicine, 37, S9-S19.
View at Publisher | View at Google Scholar - Weibel, S., Rücker, G., Eberhart, L. H., Pace, N. L., Hartl, H. M., Jordan, O. L., ... & Kranke, P. (2020). Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta‐analysis. Cochrane Database of Systematic Reviews, (10).
View at Publisher | View at Google Scholar - Cheisson, G., Jacqueminet, S., Cosson, E., Ichai, C., Leguerrier, A. M., Nicolescu-Catargi, B., ... & Benhamou, D. (2018). Perioperative management of adult diabetic patients. Intraoperative period. Anaesthesia Critical Care & Pain Medicine, 37, S21-S25.
View at Publisher | View at Google Scholar - Joshi, G. P., Chung, F., Vann, M. A., Ahmad, S., Gan, T. J., Goulson, D. T., ... & Twersky, R. (2010). Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesthesia & Analgesia, 111(6), 1378-1387.
View at Publisher | View at Google Scholar - Corcoran, T. B., Myles, P. S., Forbes, A. B., Cheng, A. C., Bach, L. A., O’Loughlin, E., ... & Ho, K. M. (2021). Dexamethasone and surgical-site infection. New England Journal of Medicine, 384(18), 1731-1741.
View at Publisher | View at Google Scholar - Tosti, R., Fowler, J., Dwyer, J., MalTenFoRT, M., Thoder, J. J., & Ilyas, A. M. (2012). Is antibiotic prophylaxis necessary in elective soft tissue hand surgery?. Orthopedics, 35(6), e829-e833.
View at Publisher | View at Google Scholar - Harness, N. G., Inacio, M. C., Pfeil, F. F., & Paxton, L. W. (2010). Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. The Journal of hand surgery, 35(2), 189-196.
View at Publisher | View at Google Scholar - Ko, J. S., Zwiebel, S., Wilson, B., & Becker, D. B. (2017). Perioperative antibiotic use in diabetic patients: a retrospective review of 670 surgeries. Journal of Plastic, Reconstructive & Aesthetic Surgery, 70(11), 1629-1634.
View at Publisher | View at Google Scholar - Yao, Z., Chen, H., Wang, X., Zhang, Y., Jian, M., Hu, J., ... & Jiang, L. (2022). Efficacy of the Short-Term versus Long-Term Administration of Antimicrobial Prophylaxis in Gastric Cancer Surgery: A Meta-Analysis of Randomized Controlled Trials. Surgical Infections, 23(7), 625-633.
View at Publisher | View at Google Scholar - Sykara, M., Maniatakos, P., Tentolouris, A., Karoussis, I. K., & Tentolouris, N. (2022). The necessity of administrating antibiotic prophylaxis to patients with diabetes mellitus prior to oral surgical procedures-a systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 16(10), 102621.
View at Publisher | View at Google Scholar - Ling, M. L., Apisarnthanarak, A., Abbas, A., Morikane, K., Lee, K. Y., Warrier, A., & Yamada, K. (2019). APSIC guidelines for the prevention of surgical site infections. Antimicrobial Resistance & Infection Control, 8(1), 1-8.
View at Publisher | View at Google Scholar - Seidelman, J. L., Mantyh, C. R., & Anderson, D. J. (2023). Surgical site infection prevention: a review. JAMA, 329(3), 244-252.
View at Publisher | View at Google Scholar - Otto-Lambertz, C., Decker, L., Adams, A., Yagdiran, A., & Eysel, P. (2023). Can triclosan-coated sutures reduce the postoperative rate of wound infection? Data from a systematic review and meta-analysis. Surgery.
View at Publisher | View at Google Scholar - Luo, W., Tao, Y., Wang, Y., Ouyang, Z., Huang, J., & Long, X. (2023). Comparing running vs interrupted sutures for skin closure: A systematic review and meta‐analysis. International Wound Journal, 20(1), 210-220.
View at Publisher | View at Google Scholar