Research Article | DOI: https://doi.org/10.58489/2836-5828/003
Modeling of Cerebral Anoxia of Respiratory Genesis in Rats
Grodno State Medical University Grodno, Republic of Belarus
*Corresponding Author: E.I. BON
Citation: М.А. FEDUTO, N. Ye. MAKSIMOVICH, S.M. ZIMATKIN, E.I. BON, А.I. GRICHENKO, I.N. BURAK, (2023). Modeling of Cerebral Anoxia of Respiratory Genesis in Rats. Archives of Urology and Nephrology. 2(1). DOI: 10.58489/2836-5828/003
Copyright: © 2023 E.I. BON, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 16 January 2023 | Accepted: 23 January 2023 | Published: 31 January 2023
Keywords: anoxia, neurons, brain
Abstract
Anoxia as an extreme degree of acute oxygen starvation of the brain cells, regardless of the causes, leads to its greatest damage. As a result of using a new model of cerebral anoxia we have established disturbances in the parietal cortex of the rat brain, manifested by changes in the size, shape of neurons, the degree of their chromatophilia. This indicates the possibility of using the method of modeling anoxia by tracheal compression to study acute cerebral oxygen deficiency. It provides an opportunity to assess visually the dynamics of neuronal changes under anoxia without shutting down the cerebral circulation. It may be of value for further study of the consequences of disorders in the brain arising from respiratory pathology and external causes accompanied by asphyxia.
Introduction
The activity of the brain in general and all specific processes in the nervous tissue depend primarily on the level of oxygen supply [2,4,5].
Anoxia as an extreme degree of acute oxygen starvation of the brain cells, regardless of the causes, leads to its greatest damage. This is due to the complexity of the morphological structure and functions performed by the brain, as well as low tolerance to oxygen deficiency, which is determined by a high metabolic level, lack of oxygen stores and macroergic compounds [3,6].
Different brain structures are differently resistant to anoxia of the same degree and duration. First of all, the functions of phylogenetically younger parts of the brain are impaired.
The main links in the pathogenesis of acute cerebral oxygen deficiency are: deficit of energy generation, disruption of potential-determining ion transport, changes in acid-base status, excitotoxicity, occurrence of oxidative stress, inflammation and apoptosis. This causes structural and metabolic disturbances in the neurons of the brain, leading to its death [1,2,3].
Studies of acute cerebral oxygen deprivation are performed on animals, more often on rats, since these processes cannot be simulated in vitro.
There are known models of cerebral anoxia by decapitation of rats or occlusion of vessels supplying blood to the brain. These methods of modeling are associated primarily with the termination of cerebral circulation [1].
However, due to the widespread respiratory pathology, as well as the possibility of external mechanical factors affecting the respiratory tract, the simulation of rat brain anoxia of respiratory genesis is relevant.
The aim of the work was to develop an adequate model of cerebral anoxia of respiratory genesis in rats.
Materials and methods of the study
The study was performed on white mongrel rats (18 males, weight 240±20 g) divided into 2 groups in compliance with the requirements of the Directive 2010/63/EU of the European Parliament and of the Council dated September 22, 2010 on the protection of animals used for scientific purposes.
The control group (n=6) consisted of falsely operated rats with reproduction of all stages without anoxia.
In the second group of rats (n=12), divided into 2 subgroups (n=6), we simulated respiratory anoxia by tracheal compression. For this purpose, after achieving the required depth of anesthesia (sodium thiopental, 40 mg/kg), the animal was fixed on the preparatory table with abdomen upwards. After cutting the skin along the midline of the neck from the chin area to the sternum, the neck muscles were bluntly loosened to the trachea, on which a ligature was applied for 30 minutes (subgroup 1) and 60 minutes (subgroup 2) below the rharyngeal cartilage.
After decapitation, the brain was quickly extracted and fixed in Carnois fluid. The brain sections (parietal lobe) were then dehydrated in increasing concentrations of ethanol and embedded in paraffin. Frontal paraffin sections of the parietal lobe 7 µm thick were prepared and stained with Nissl thionine. The localization of the parietal cortex was determined using a stereotactic atlas [9].
Visual and morphometric evaluation of neurons was performed using ImageWarp image analysis software (Bitflow, USA).
Thirty neurons of the fifth layer of the parietal cortex were studied in each animal to determine their size, shape and degree of chromatophilia [7,8].
The obtained quantitative continuous data were processed using methods of nonparametric statistics, the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). The data were presented as Me (LQ; UQ), where Me was the median; LQ was the lower quartile value; UQ was the upper quartile value. The differences between the indices of control and experimental groups were considered reliable at p<0>
Results of the study
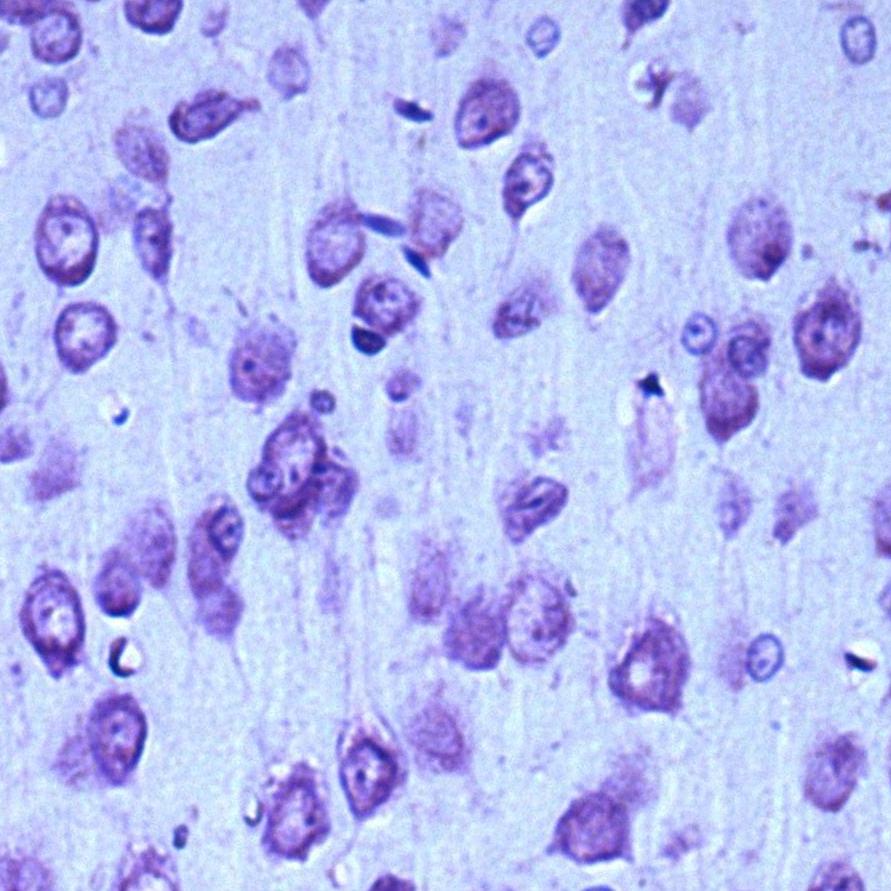
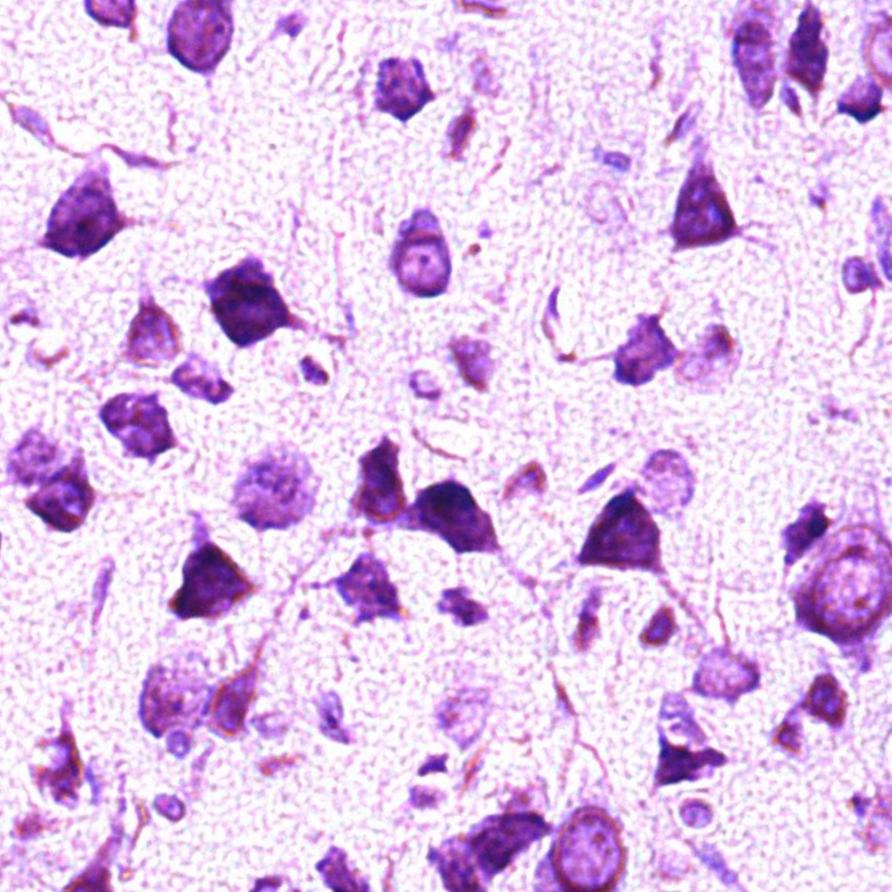
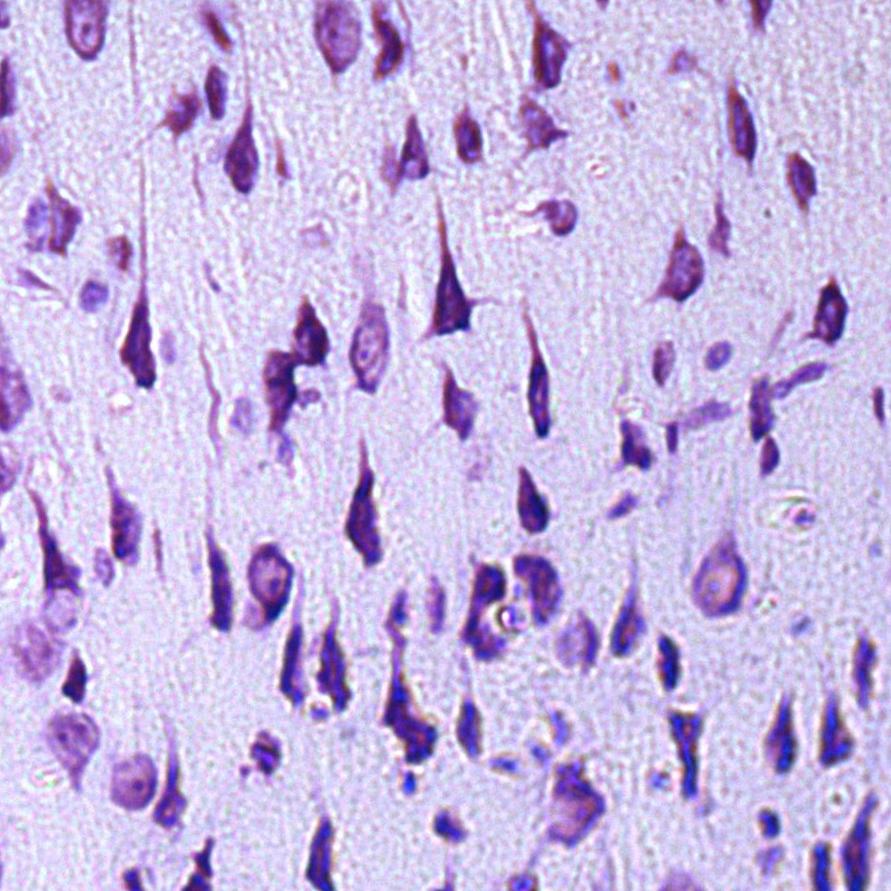
In the control group, up to 95% of the population of parietal cortex neurons were normochromic cells, while the remaining neurons were hypochromic (4%) and hyperchromic wrinkled (1%) cells (Fig. 1).
Fig. 1. Neurons of the fifth layer of the rat parietal cortex. Digital micrograph. Nissl staining.
A – control group (predominance of normochromic neurons); B – after 30 minutes of anoxia (predominance of hyperchromic wrinkled neurons); C – after 60 minutes of anoxia (predominance of hyperchromic wrinkled neurons);
Std. lens x 20.
Changes in the size and shape of neurons in the parietal cortex, as well as in the intensity of their cytoplasm staining were established in the experimental group animals.
In both studied time periods in rats with anoxia there dominated hyperchromic wrinkled neurons - elongated and polygonal neurons with intensely stained cytoplasm, about 75% - in the group of rats with 30 min anoxia (p<0>
There was no change in neuronal area after 30 minutes of anoxia (p>0.05), Fig. 2, Table 1. But there was a change in the shape of neurons: the form factor decreased by 23% (p<0>
Table 1 - Indices of size and shape of parietal cortex neurons in rats with anoxia
| Groups | Indicators | ||
| Area (µm2) | form-factor (units) | elongation factor (units) | |
| Control | 186,6 (180,2; 191,0) | 0,90 (0,90; 0,90) | 1,25 (1,16; 1,34) |
| Anoxia 30 minutes | 180,6 (167,5; 188,7) | 0,70* (0,68; 0,71) | 2,13* (2,04; 2,23) |
| Anoxia 60 minutes | 121,5*(118,6; 133,4) | 0,63* (0,59; 0,67) | 2,19* (2,11; 2,27) |
Note: * - differences in reliability compared to the control group (p<0>
By 60 minutes of anoxia the neuronal area had decreased by 35% (p<0>0.05).
Thus, a study of the effects of respiratory anoxia on parietal cortex neurons in rats modeled by tracheal compression revealed the presence of structural changes that were more pronounced after 60 minutes.
Conclusion
As a result of using a new model of cerebral anoxia, abnormalities in the parietal cortex of the rat brain, manifested by changes in the size, shape of neurons, the degree of their chromatophilia, were established. This indicates the possibility of using the method of modeling anoxia by tracheal compression to study acute cerebral oxygen deficiency. It provides an opportunity to visually assess the dynamics of neuronal changes under anoxia conditions without shutting down the cerebral circulation. The proposed method of modeling cerebral anoxia in rats by tracheal compression may be of importance for further study of the consequences of brain disorders arising from respiratory pathology and external causes accompanied by asphyxia.
References
- Bon EI, (2018), Modeling techniques and morphofunctional markers of cerebral ischemia / Bon EI, Maksimovich NYe // Biomedicine. - № 2. - p. 59-71.
View at Publisher | View at Google Scholar - Shin TH, Lee DY, Basith S, Manavalan B, Paik MJ, et al. (2022) Metabolome Changes in Cerebral Ischemia. Cells, 9 (7): 1630.
View at Publisher | View at Google Scholar - Sylvestrea DA, Otoki Y, Metherel AH, Bazinet RP, Slupsky CM, et al. (2022) Effects of hypercapnia / ischemia and dissection on the rat brain metabolome/ Neurochemistry International.
View at Publisher | View at Google Scholar - Koizumi S, Morizava YM (2018) New roles of reactive astrocytes in the brain; an organizer of cerebral ischemia. Neurochemistry International, 107 p.
View at Publisher | View at Google Scholar - Zhao Y, Zhang X, Wei Y (2022) Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). International of Molecular Medicine, 49 (2): 15.
View at Publisher | View at Google Scholar - Sabeeha, Hasnain SE (2019) Forensic Epigenetic Analysis: The Path Ahead. Medical Principles and Practice, 301 p.
View at Publisher | View at Google Scholar - Ma Z, Ma Y, Zhang N (2018) Development of brain-wide connectivity architecture in awake rats. Neuroimage, 176: 380-389/
View at Publisher | View at Google Scholar - Paxinos, G. The rat nervous system / G. Paxinos // Amsterdam – Boston – Heidelberg – London - New York – Oxford – Paris – SanDiego – SanFrancisco – Singapore – Tokyo: Elsevier Acad. Press. – 2004. – 1035 p.
View at Publisher | View at Google Scholar - Paxinos, G. The Rat Brain in stereotaxis coordinates / C. Watson // Academic Press, Australia. – 1998. – 242 p.
View at Publisher | View at Google Scholar - Brown BM, Newcombe RG (2009) Non-null semi-parametric inference for the Mann-Whitney measure. Journal of Nonparametric Statistics, 743 p.
View at Publisher | View at Google Scholar