Research Article | DOI: https://doi.org/10.58489/2836-6387/002
First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy in elderly Patients with advanced lung adenocarcinom in routine clinical practice-results from A Subgroup Analysis
- MPH,Department of Oncology,China Raditional Protection Research Institute Hospital,Tai Yuan City, Shan Xi Province,030006,China.
- MD, Department of Oncology,Shan-Xi Bethune Hospital,Taiyuan City,Shanxi Province,China.
*Corresponding Author: An-Tai He
Citation: An-Tai He, Yi Pei. (2022). First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy in elderly Patients with advanced lung adenocarcinom in routine clinical practice-results from A Subgroup Analysis, Journal of Virology and Vaccination 1(1): DOI: 10.58489/2836-6387/002
Copyright: © 2022 Z An-Tai He, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 27 July 2022 | Accepted: 12 September 2022 | Published: 26 December 2022
Keywords: lung adenocarcinoma, EGFR inhibitor,Cytotoxic chemotherapy, Osimertinib.
Abstract
The third generation Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor (TKI) osimertinib has been initially approved for T790M positive lung adenocarcinoma patients and more recently for first-line treatment of EGFR-mutant T790M negative lung adenocarcinoma, Similarly to previous generation TKIs, despite the high response rate, disease progression eventually occurs and current clinical research is focused on novel strategies to delay the emergence of osimertinib resistance.In this study,we investigated as the combination of osimertinib/ gefitinib/ erlotinib with cytotoxicchemotherapy for
EGFR-mutated positivelung adenocarcinoma patientsin long-term survivaloutcomes.
Materials and Methods:
Weenrolled Ⅲb-IV stage lung adenocarcinoma patients with an EGFR mutation,Patients receivingstandard Osimertinib,Gefitinib,Erlotinip alone treatment and Osimertinib,Gefitinib and Erlotinip with cytotoxic
chemotherapy treatment were retrospectively reviewed.The performance status were collected,The response rate, progression-free survival(PFS) and overall survival (OS) and toxicity profile were analyzed.
Results:
Between January 2014 to Dec 2020,240 patients with Ⅲb-Ⅳstages lung adenocarcinoma were enrolled from an institution.All patients who received different standard treatment respectively,were divided into four groups,64 who received(gefitinib or Erlotinb)with cytotoxic chemotherapy, 60 who received singlegefitinib or erlotinib.58 who received (Osimertinib) with cytotoxic chemotherapy,58 who received single (Osimertinib) were eligible for this study.First generation Chemical-TKItherapy group PFS vs First generation TKI therapy alonePFS.P<0>
16.00 month.95%CI [11.98,20.01]. First generation Chemical-TKItherapy group OS vs First generation TKI therapy alone OS. P<0>
Chemical-TKItherapy group PFS vs.First-Third generation TKI therapy alone PFS.P<0>
significant prognostic factors for OS were old age (55-69 years) (HR = 0.49 [0.28–0.89], p < 0.02) and gene mutation (Positive) (HR = 0.15 [0.07–0.29], p < 0.05), First add third generationTKI with chemicaltherapy (HR = 0.56 [0.35–0.89], p < 0.02).
Conclusion:
First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy represents a suitable palliative treatment option in further therapy lines for elderly patients with advanced lung adenocarcinoma.The results obtained under real-life conditions add to our understanding of the benefitsand risks of First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy in routine clinical practice.
Background:
Lung cancer is the leading cause of cancer deaths worldwide [1], about 85% of cases are diagnosed as non-small-cell lung cancer (NSCLC)[2].The medianage of NSCLC patients is 70 years and the disease is
usually diagnosed in advanced stages,when curative surgery is no longer feasible [3]. In metastasized disease, first-line chemotherapy is often not successful and the 5-year survival rate is only 4.2% [3]. NSCLC is histologically classified into the major subtypes adenocarcinoma (~ 40%) [4, 5],Recurring mutations have been reported in genes coding for epidermal growth factor receptors (EGFR) in 10–40% of adenocarcinomas [6,7,8], EGFR mutations can lead to constitutive activation of anti-apoptotic and proliferation signaling pathways,which promote cancer progression [9],EGFR tyrosine kinaseinhibitors (TKI) are the preferred
first-line treatment for advancedNSCLC with EGFR mutations [10, 11], TreatingNSCLC is challenging
because of the advanced age of patients.As EGFR-TKI avoid the systemic side effects of traditional chemotherapy,they might be more suitable for treating elderly patients [12]. Osimertinib, a third-generation EGFR-TKIthat selectively binds the C797 residue inhibiting the T790M mutation, has shown high activityin term of Progression-Free Survival (PFS) and overall response rate in EGFR-T790M positive patients [13, 14] and efficacy superior to gefitinib/erlotinib in the first-line treatment by approximately a 9 months advantage in PFS [15]. However,acquired resistance occurs also to osimertinib either in T790M-positive NSCLC patients or in patients treated in first-line [16, 17].EGFR-dependent or independent mechanisms of resistance have been described even if they remain not completely understood [16]. EGFR G796/C797, L792 and L718/G719 mutations, MET and HER2 amplification, BRAF, KRAS, and PIK3CA mutations, oncogenic fusion mutationsin FGFR3, RET, and NTRK were recently identified in a large cohorts of osimertinib-resistant lung cancer patients either treated in second-line [18, 19] and in first-line [20].Knowledge of these mechanisms is relevant in order to develop new therapeutic strategies to overcome TKI-resistance; however, how prevent or delay the acquisition of resistance remains an important issue.Some data indicated that in PC9 cell line and xenograft models, the combination of gefitinibwith pemetrexed or the intermittent combination of pemetrexed and gefitinib prevented some the appearance of gefitinib resistance mediated by T790M mutation and epithelial-mesenchymal transition [21]; however,the combination was ineffective when gefitinib was administered before
pemetrexed.Theoretically,Chemotherapy,given its different and more generic mechanism of action,can postpone the resistance to EGFR-TKIs by limiting the tumor heterogeneity,thus improving the efficacy of treatment either in first-and second-line.Osimertinib combined or intercalated with chemotherapy deserves to be considered either for patients in progression after first/second-generation TKIs or in first-line setting.Our study was undertaken to explore a long-term survivaloutcomes in the combination of osimertinib with pemetrexed add platinum and the combination of gefitinib/erlotinib with pemetrexed add platinum in elderly lung adenocarcinoma patients.
Methods:
Patients
Methods Between January 2014 and Dec 2020, 240 patients were diagnosed in Shan-Xi Bethune Hospital,Taiyuan City,China.
All patients were aged between 55 and 84 years old. Inclusion criteria were as follows:(1) Pathological diagnosis of lung adenocarcinoma; (2) Karnofsky performance score >60; (3) Adequate organ (white blood cell > 4.0×10/L; neutrophil > 2.0×10/L; hemoglobin > 90 g/L; platelet> 100×109/L; aspartate
aminotransferase/alanine transaminase < 2>
Treatment method:
Table 2. Chemotherapy:(1) pemetrexed plus carboplatin or cisplatin. (2) Docetaxel plus carboplatin or cisplatin.and so on.Chemotherapy used for 4 to 6weeks or more.TKI therapy: Before TKI therapy, Tumor gene mutation profile,including EGFRT790M,ALK-M, KRAS-M,METM,,RETM,ROS,and so on gene, was performed.If the test was positive,first-generationTKI therapy drugs,Gefitinib, Erlotinib,Ectinib were used.After the first-generation drugsshowed resistance,Third-generation TKI therapy drug Osimertinib was used.Eligible patients were randomized to oneof the following treatment arms:240 patients divided into 2 group.First group 124patients.60 patients alone Gefitinib group 250 mg/ each 1/ d, or Erlotinip group 150 mg, each 1/ d, oral administration.Oral administration until disease progression.64
patients,Gefitinib/ Erlotinip with chemotherapy group,Chemotherapy regimen:intravenously administered pemetrexed sodium on day 1 of each cycle,500mg/m2,dose.Cisplatin was given intravenously on days 2,3 and 4, 30 mg /m2,dose or carboplatin on day 1,The doses were 10 mg /m2,One cycle continuous treatment for 4~6 cycles or more.pemetrexed 175 mg/m2,and carboplatin 10 mg /m2, administered intravenously on day.intercalated with Gefitinib 250 mg group or Erlotinip group 150 mg orally on days until progressive disease,or until a discontinuation criterion was met.Second group116 patients,58 patients alone osimertinib group 80 mg/ d,Oral administration until disease progression.58 patients Osimertinib targeted therapy with chemotherapy,Chemotherapy regimen was same asfirst Group,intercalated with osimertinib group 80 mg/ d,Oral administration until disease progression.
Evaluation:
Tumor response was assessed as complete response (CR), partial response (PR), stable disease (SD), or progression disease (PD) in accordance with the standard of RECIST [22]. A CR was defined as the complete disappearance of all clinically detectable tumors for at least 4 weeks. A PR was defined as an at least 30
Statistical analyses:
The incidence of time-to-event data in different subgroups was analyzed using the Kaplan-Meier method and compared with the log-rank test.The potential factors,survival and response data were analyzed overall and in the following subgroups:age (55–69 or ≥ 70 years),EGFR mutation (positive or negative) and
gender,metastatic lesions1-2 or ≥3.Treatment method(TKI-chemicaltherapy,or TKI therapy aloneadd
chemical therapy alone). the OS was additionally investigated using Cox regression models (considering single and multiple factors). Multivariable Cox regression analyses were used to estimate the HR and 95% CI for the relationship between the characteristics and overall survival.Statistical analyses were performed using SPSS (Mac ver. 21.0, IBM Corp.).All statistical tests in our study are 2-tailed.A p-value of less than 0.05 is considered statistically significant.
Results
Table 1 Patientbaseline characteristics (N = 240)
| Characteristic | No. (%) | ||||
| Age (yr) | |||||
| 55-69 | 152(63.15) | ||||
| ≥ 70 | 88(36.84) | ||||
| Gender | |||||
| Male | 107(44.73) | ||||
| Female | 133(55.26) | ||||
| Gene mutation (Tested) | |||||
| EGFR + | 100(41.44) | ||||
| EGFR - | 57(23.68) | ||||
| Wild-type | 5 (1.97) | ||||
| T790M mutations + | 44(18.42) | ||||
| ALK mutations+ | 6 (2.63) | ||||
| KRAS mutations+ | 13(5.26) | ||||
| RET mutations+ | 8(3.28) | ||||
| MET mutations+ | 8 (3.28) | ||||
| EGFR gene mutation site-n | |||||
| Exon18 | 11 (4.76) | ||||
| Exon19 | 57(23.80) | ||||
| Exon19 + Exon21 | 4(1.58) | ||||
| Exon 20 + | 8(3.17) | ||||
| Exon21L858 | 61(25.39) | ||||
| Chemical-TKI therapy | |||||
| Yes | 122(50.83) | ||||
| No | 118(49.16) | ||||
| First-generation Chemical-TKI therapy | 64(26.66) | ||||
| First-third generation Chemical-TKI therapy | 58(24.16) | ||||
| TKI therapy alone | |||||
| Yes | 118(49.58) | ||||
| No | 122(50.83) | ||||
| First generation TKI therapy alone | 60(25.00) | ||||
| First-third generation TKI therapy alone | 58(24.16) | ||||
Table 2. Drugs administered as First generationTKI therapy alone,First generation Chemical-TKI therapy,
First-third generation TKI therapyalone,First-third generation Chemical-TKItherapy.
| Gefitinib+Etoposide VP16+Cisplatin | 13 | 5.26 % |
| Gefitinib+Bevacizumab+ Cisplatin+Pemetrexed | 10 | 3.94 % |
| First-third generation TKI therapy alone | 52 | 21.4 9% |
| Type of treatment | ||
| Osimertinib+Gefitinib | 25 | 10.5 2% |
| Osimertinib | 26 | 10.9 6% |
| First-third generation Chemical-TKItherapy | 58 | 24.1 2% |
| Type of treatment | ||
| Osimertinib+Gefitinib+Docetax el+Carboplatin | 21 | 8.77 % |
| Osimertinib+Erlotinib+Pemetre xed+Cisplatin | 16 | 6.57 % |
| Osimertinib+Pemetrexed+Cispla tin | 11 | 4.38 % |
| Osimertinib+Docetaxel+Carbopl atin | 11 | 4.38 % |
Six or more cycles of chemotherapy were completed in 95% of patients and only one cycle was completed in 4.5% of patients.
Table 3. Results of Cox univariate and multivariate regression analysis
| Characteristic | Univariable analyses (95% CI) | Hazard ratio p-value | Univariable analyses (95% CI) | Hazard ratio p-value |
| Age (yr) | ||||
| 55-69 | Reference | Reference | ||
≥70 | 0.49(0.30-0.81) | 0.005 | 0.49 | 0.02 |
| (0.28-0.89) | ||||
Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.77(0.53–0.96) | 0.04 | 0.92(0.56- 1.53) | 0.76 |
| Gene Mutation | ||||
No |
Reference |
Reference | ||
Yes |
0.72(0.07-0.25) |
0.01 |
0.15(0.07- 0.29) |
0.01 |
First add thirdgeneration TKI therapy with Chemical therapy | ||||
No | Reference | Reference | ||
Yes | 0.56(0.35-0.93) | 0.02 | 1.50(0.42- 5.31) | 0.52 |
|
|
| ||
CI.Confidence interval.Cox regression models with adjustment for single factors showed a significant influence of age (yr)(p = 0.005),gender (p = 0.04) and EGFR status (p = 0.01),first add third generationTKIherapy with chemicaltherapy (p = 0.02) on OS. Accordingly, Age (yr)55-69 had an 51% reduced risk of death compared to≥70 (yr) (hazard ratio([HR] 0.49, 95% CI 0.30–0.81). Females had an almost 30% reduced risk of death compared to males (hazard ratio([HR] 0.71, 95% CI 0.53–0.96). Patients with an EGFR mutation had an almost 28% reduced risk of death compared to negative patients([HR] 0.72,95% CI 0.07-0.25). First add third generationTKItherapy with chemicaltherapy had an almost 54% reducedrisk of death compared to first add third generation TKItherapy alone.
Figure 2
Figure2 A.First generation Chemical-TKItherapy group PFS vs First generation TKI therapy alone PFS. P<0> Figure2 B.1.First generation Chemical-TKItherapy group OS vs First generation TKI therapy alone OS. P<0>
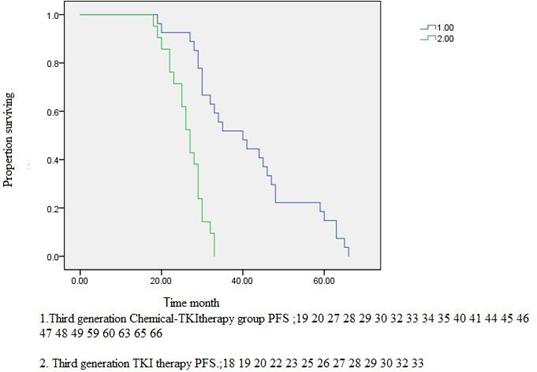
Figure2C.1.Third generation Chemical-TKItherapy group PFS vs Third generation TKI therapy PFS. P<0>
95%CI [24.77,29.22].
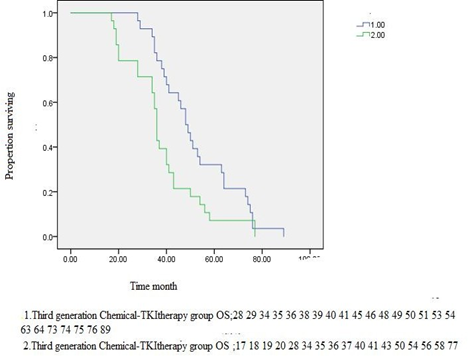
Figure2D.1.Third generation Chemical-TKItherapy group OS vs Third generation TKI therapy OS. P<0>
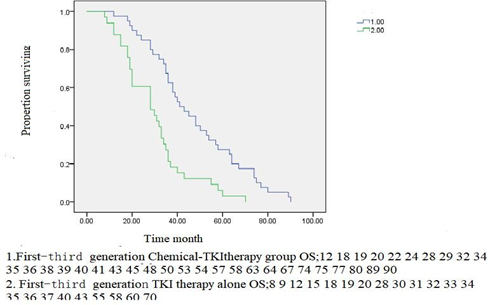
Figure2E1.First-Third generation Chemical-TKItherapy group PFS vs.First-Third generation TKI therapy alone PFS. P<0>
Figure2F.1.First-Third generation Chemical-TKItherapy group OS vs First-Thirdgeneration TKI therapy alone OS. P<0>
Table 4:
Clinical endpoints and Treatment outcomes:TKItherapy with Chemical-TKLtherapy and TKLtherapy alonestratified by patientbaseline characteristics, for the overallpatients with Lung adenocarcinoma.
Response, N (%) | First generation | First generation | p-value | Third generation Chemical- | Third generation TKItherapy | p-value |
Chemical- TKItherapy (N = 64) (%) | TKItherapy alone (N = 60) (%) | TKItherapy (N = 58) (%) | alone (N = 58) (%) | |||
| Partial response (PR) | 45 (70.23) | 33 (55.23) | 47 (80.23) | 43(73.45) | ||
Stable disease (SD) | 21(32.72) | 12 (20.12) | 28(48.64) | 24(41.17) | ||
| ≥ 6 weeks | ||||||
Progressive disease (PD) | 3(5.41) | 6 (10.34) | 2(3.11) | 3(5.12) | ||
ORR (CR+PR) | 49 (77.12) | 29(47.45) | <0> | 52(89.34 | 44(75.43) | <0> |
| DCR (CR+PR+SD) | 54 (84.34) | 41(67.54) | <0> | 55(95.34) | 46 (79.21) | <0> |
| Survival time | ||||||
PFS, months, median (95% CI) | 22.00 (16.29- 27.70) | 16.36(11.98- 20.01) | <0> | 40.00(28.12- 51.87) | 27.00(24.78- 29.22) | <0> |
OS, months, median (95% CI) | 32.00(25.29- 38.71) | 28.00(14.58- 41.42) | <0> | 48.00(42.81- 53.18) | 36.00(34.72- 50.27) | <0> |
ORR, overall response rate; CR, complete response; DCR, disease control rate;CI, confidence interval;PFS, progression-free surviv for treatment; OS, overall survivalfor treatment.
Table 5.Summary of the most common adverseevents for the overallpatients with Lung adenocarcinoma.
Summary of the most common adverse events for the overall patients with Lung adenocarcinoma. Adverse event withAE (Grade 1-4) | First generation Chemical-TKIth erapy (N = 64) (%) All grade | First generation TKItherapy alone(N = 60) (%) All grade | Third generation Chemical-TKIth erapy (N = 58) (%) All grade | Third generation TKItherapyalone (N = 58) (%) All grade |
| Skin rash | 40 (63.12) | 37(61.48) | 34(58.32) | 31(53.02) |
| Anorexia | 34(53.12) | 25(42.21) | 25 (42.43) | 18 (31.81) |
| Cough | 30(46.12) | 25(40.23) | 21 (36.23) | 19(33.31) |
| Nausea | 28(43.12) | 18(30.23) | 18(30.12) | 14(24.21) |
| Fatigue | 19 (29.09) | 17(27.58) | 9 (15.13) | 6(10.61) |
| Diarrhea | 30(47.12) | 26(43.34) | 24 (41.12) | 18(31.81) |
| Neutropenia | 21 (32.12) | 16(27.12) | 15 (26.12) | 11(18.22) |
| Anemia | 28 (43.00) | 21(35.12) | 19 (32.12) | 16(27.31) |
| Thrombocytopenia | 26(40.07) | 19(32.12) | 19 (32.12) | 16 (27.31) |
| Increased LFT | 35 (54.54) | 25(41.72) | 11(18.13) | 8(13.61) |
| Mucositis | 12 (18.18) | 12(20.68) | 9(15.23) | 0(0.00) |
AE adverse event; Gr grade; N number,LFT liver functiontest
During the study,794 AEs were observed in 240 patients (Table 5). According to the common toxicity criteria for adverse events(CTC), The most commonly reportedAEs were rash and anorexia,diarrhea followedby increased LFT, cough,nausea,
anemia and neutropenia. Most of the toxicity was grade 1 to 2, and remitted after treatment. The frequency of AEs was not significantly affected by age or EGFR mutation status (data not shown). All AEs reportedwere consistent with those
described in the summary of product characteristics [23].
Discussion
The study was designed to evaluate the effect of intercalation therapy with gefitinib or erlotinib or osimertinib with platinumar add pemetrexed chemotherapy.Our first generation target group includes gefitinib,erlotinib.The study demonstin relation to PFS, and OS.Toxicity profiles were generally clinicallytolerabled. In another studies are same[21-25],the sequence-dependent synergism between platinumar add pemetrexed and gefitinib was demonstrated in human lung cancer cell lines with both wild-type and mutant EGFR genes [26].The concurrent regimen is currently being evaluated against gefitinib alone in a randomized phase III study recently presented at ESMO 2018 meeting [2].In this trial, the patients who receiveda combination of gefitinib with carboplatin-pemetrexed showeda statistically significant benefit in survival (PFS of 20.9 vs 11.2 months, p < 0.001 and OS of 52.2 vs 38.8, p = 0.013 for gefitinib and carboplatin/pemetrexed and for gefitinib alone, respectively).Several later phase I/II clinical studies showed that an intercalated regimen of chemotherapy and EGFR TKI were safe and effective [25–28, 29].WSW clinical studies reported that the intercalated regimen offered superior efficacy compared to chemotherapy or EGFR TKIs alone [30, 31]. In a three-arm phase II study,The combination was suggested as a new treatment option for patients with unknown EGFR status in a previous clinical study [30],Although molecular tests are used routinely in clinical practice, EGFR status remains unknown in certain patients.We think that the intercalated strategy could be effective in patients with wild-type or unknown EGFR status.According to several clinical studies, Intercalated treatment might be a promising approach for patients with lung adenocarcinoma with EGFR mutant disease or selected patient with unknown EGFR mutation status, [30–32].Our results are first generation Chemical-TKItherapy group PFS vs first generation TKI therapy alone PFS. P<0>
First generation Chemical-TKItherapy group OS vs First generation TKI therapy alone OS. P<0>
Chemical-TKItherapy group vs First generation TKI therapy alone had a stronger effect on ORR and DCR.Osimertinib is a third-generation EGFR TKI,A large rando mized trial comparing osimertinib to gefitinib or erlotinib reported that PFS was significantly longer in the osimertinib arms, and time to CNS metastases was significantly delayed because osimertinib crosses the blood-brain barrier.[33] Toxicity rateswere lower with osimertinib than the first-generation inhibitors and the HRs for benefit were similar in younger and older patients. Similarly to previous generation TKIs, despite the high response rate,disease progression eventually occurs and current clinical research is focused on novel strategies to delay the emergence of osimertinib resistance.Although preclinical and clinical researches have explored the
interaction of first-generation EGFR-TKIs and cytotoxic agents [ 34,35,36,29,30,31,32], to date there are no data on clinical combination of chemotherapy with third-generation EGFR-TKIs, suc as osimertinib. In this study, we explored the efficacy of osimertinib combined with pemetrexed add platinumar in lung adenocarcinoma.A strong anti-tumor effect was observed when osimertinib was combined with pemetrexed add platinumar intercalated,By contrast osimertinib monotherapy.We strongly indicating that the addition of chemotherapy may potentiate the efficacy of osimertinib either in term of inhibition of tumor growth or appearance of relapses.Figure2C.1.Third generation Chemical-TKItherapy group PFS vs Third generation TKI monotherapy PFS. P=0.005. Mean Survival Time 40.73,95%CI [33.56,47.90] VS 26.66 95%CI [22.89,30.44]. Figure2D.1.Third generation Chemical-TKItherapy group OS vs Third generationTKI monotherapy OS. P=0.04.Mean Survival Time 54.00,95%CI[45.81,62.18] VS 39.72 95%CI[29.18,50.27].Table 4.Third generation Chemical-TKItherapy group vs Third generation TKI therapy alone had a stronger effect on ORR and DCR.In a mouse model of PC9T790M xenograft tested in vitro,A strong anti-tumor effect was observed when osimertinib was combined with pemetrexed or cisplatin intercalated with osimertinib alone, no tumor became resistant, differently from the treatment with osimertinib alone which induced acquired resistance in 50% of mice.The combination treatment enhanced the percentage of fibrotic tissue within the xenograft tumors and the small tumors did not regrow when the administration of drugs was stopped, indicating a stronger efficacy in eradicating parenchymal tumor cells [39]. In PC9 and PC9T790M cell lines, analysis of signaling transduction pathways and protein related to cell death revealed that the combination treatment did not affect the intracellular transduction pathways,which were already completely suppressed by osimertinib alone,but strongly enhanced apoptosis signaling via caspase-7 activation.This observation may be of relevance for the results obtained in vivo.therefore, the selective pressureexerted by TKIs may promote the clonal expansion of resistant clones through different molecular mechanismsresults[37,38].Our also provide a strong rationale for randomizedstudies comparing osimertinib monotherapy vs osimertinib plus chemotherapy, either in EGFR T790M positive and negative in EGFR-TKI naïve NSCLC patients. A phase III trial evaluating osimertinib combined with platinum-pemetrexed vs osimertinib monotherapy could be the right step forward to significantly prolong the survival of EGFR-mutated NSCLC patients[40].Combination cisplatin/carboplatin plus pemetrexed is the standard treatment regimen for advanced nonsquamous NSCLC and has been frequently used as the backbone of combination treatment[41,42,43].After eradicating tumors with heterogeneity, adding chemotherapy to osimertinib might increase the response rate and improve PFS and OS with a low incidence of grade ≥ 3 AEs [44],Table5.For each of these AEs,the majority of Osimertinib
with carboplatin-pemetrexed chemotherapy were grade 2 or 3 in severity,mild toxicities including skin rash (58.32%), anorexia (42.43%), nausea (31.12%), diarrhea (41.12%), cough (36,23%), anemia (32.12%),
thrombocytopenia (32.12%) events.Less than the commonadverse effects of first generation
Chemical-TKItherapy group.Our Cox multivariate analysis also showed that age ≥70 years (in contrary to 55-69 years),mutation of genes positive compared to negative),Females compared to males,TKItherapy with chemicaltherapy compared to TKItherapy alone and chemicaltherapy alone all were significant prognostic factors.seeTable3.A large phase-3 trial with erlotinib including 586 younger and 163 elderly patients demonstrated a similar survival and quality of life (QoL) in both age groups, although a somewhat higher toxicity in the elderly was observed [45]. Clinical studies examining the elderly population are limited and often firm conclusions cannot be drawn [46,47]. In accordance with previous findings, females treated with erlotinib lived longer than males [48,49]. OS was significantly better in females than males (p = 0.04). Gene mutation improved survival time in patients. Lung adenocarcinoma with EGFR mutations was found to be 41.44% in this study.Recurring mutations have been reportedin genes codingfor epidermal growth factor receptors (EGFR) in 10–40% of adenocarcinomas [50,51,52], The mutant patients had a longer overall survival (OS) than the wild-type patients[54],Our patient with positive EGFR gene mutations demonstrated a longer progress-free OS survival than those with negative and wild-type gene.
Nevertheless,EGFR mutations were more frequent in patients over 75 than in younger patients:17% versus 8.1% (P<0>
Conclusion
First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy represents a suitable palliative treatment option in further therapy lines for elderly patients with advanced lung adenocarcinoma.The results obtainedunder real-life conditions add to our understanding of the benefitsand risks of First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy in routine clinical practice.
Abbreviations
NSCLC:
Non-small-cell lung cancer
EGFR:
epidermal growth factor receptors
TKIs:
tyrosine kinase inhibitors
AE:
Adverse event
CI:
Confidence interval
DCR:
Disease control rate
ORR:
Objective response rate
CR:
complete response
PR;
partial response,
SD
stable disease
PD
progression disease
HR:
Hazard ratio
OS:
Overall survival
PFS:
Progression-free survival
QoL:
Quality of life
Declarations
- Ethicalstatement
All patients signed informed consent before treatment, including their consent to treatment and clinical information for further prognostic factors analysis.This study was approved by the Research Ethics Committeeof Shan-Xi BethuneHospital,Taiyuan City,Shanxi Province,China
- Consent for publication
Wewould like to submit the enclosed manuscript entitled “First-Third generation EGFR inhibitor combined with cytotoxic chemotherapy in elderly Patients with advanced lung adenocarcinom in routine clinical practice-results from A Subgroup Analysis. we wish to be considered for publication in this Journal, no conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. We would like to declare on behalf of our co-authors that the work described was original research that has not been published previously and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
The Authors: Ming-Wei Chen, MD1, An-Tai He, MPH2, Yi Pei, MD.
- Availability of data and material:All data, models, and code generated or used during the study appear in the submitted article.The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
- Competing interests,Funding:
This research received no grant from any funding agency in the public commercial or not-for profit sectors. The authors declare that there is no conflict of interest
- Authors' contributions:
Chen, contributed to the conception of the study; He, performed the experiment;Chen, He,contributed significantly to analysis and manuscript preparation;He,performed the data analyses and wrote the manuscript; Yi Pei, helped perform the analysis with constructive discussions. Acknowledgements Firstly, I would like to give my sincere gratitudeto Prof.fu-bin Qiou my tutorwho, with extraordinary patienceand consistent encouragement, gave me great help by providing me with necessary materials, advice of great value and inspiration of new ideas. It is his suggestions that draw my attention to a number of deficiencies and make many things clearer. They graciously make considerable comments and sound suggestions to the outlineof this paper.It is of great help for me to finish this thesis successfully.
The authors An-tai He
References
- Torre LA,Bray F,Siegel RL,Ferlay J,Lortet-Tieulent J,Jemal, (2012),( 2015), A.Global cancer statistics,. CA Cancer J Clin.;65(2):87–108.
View at Publisher | View at Google Scholar - American Cancer Society:https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Accessed 19 Mar 2018. 3.
View at Publisher | View at Google Scholar - SEER Cancer Statistics: http://seer.cancer.gov/statfacts/html/lungb.html. Accessed 2 Dec 2015 4.
View at Publisher | View at Google Scholar - Travis WD, (2011) Pathology of lung cancer. Clin Chest Med.;32(4):669–92.
View at Publisher | View at Google Scholar - Chang JS, Chen LT, Shan YS, Lin SF, Hsiao SY, Tsai CR, Yu SJ, Tsai HJ, (2015)Comprehensive analysis of the incidence and survival patterns of lung Cancer by Histologies, including rare subtypes, in the era of molecular medicine and targeted therapy: a nation-wide Cancer registry-based study from Taiwan. Medicine (Baltimore).;94(24): e969.
View at Publisher | View at Google Scholar - Olszewski AJ, Ali S, Witherby SM, (2015) Disparate survival trends in histologic subtypes of metastatic non-small cell lung cancer: a population-based analysis. Am J Cancer Res.;5(7):2229–40.
View at Publisher | View at Google Scholar - Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Larussi T, Mucilli F, Mezzetti A, (2005)et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol.;23(4):857–65.
View at Publisher | View at Google Scholar - Sugio K, Uramoto H, Ono K, Oyama T, Hanagiri T, Sugaya M, Ichiki Y, So T, Nakata S, Morita M, (2006) et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer.;94(6):896–903.
View at Publisher | View at Google Scholar - Varghese AM, Sima CS, Chaft JE, Johnson ML, Riely GJ, Ladanyi M, Kris MG, (2013) Lungs don't forget: comparison of the KRAS and EGFR mutation profile and survival of collegiate smokers and never smokers with advanced lung cancers. J Thorac Oncol.;8(1):123–5.
View at Publisher | View at Google Scholar - Yarden Y, Sliwkowski MX, (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol.;2(2):127–37.
View at Publisher | View at Google Scholar - Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, (2016) et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol.;27(suppl 5): v1–v27.
View at Publisher | View at Google Scholar - https://europepmc.org/article/med/28958503
View at Publisher | View at Google Scholar - Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, (2017) et al. Osimertinib or platinum-Pemetrexed in EGFR T790M-positive lung Cancer. N Engl J Med.; 376:629–40.
View at Publisher | View at Google Scholar - Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, (2015) et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med.; 372:1689–99.
View at Publisher | View at Google Scholar - Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, (2018) et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung Cancer. N Engl J Med.; 378:113–25.
View at Publisher | View at Google Scholar - Minari R, Bordi P, Del Re M, Facchinetti F, Mazzoni F, Barbieri F, Camerini A, Comin CE, Gnetti L, Azzoni C, (2018) et al. Primary resistance to osimertinib due to SCLC transformation: issue of T790M determination on liquid re-biopsy. Lung Cancer.; 115:21–7.
View at Publisher | View at Google Scholar - Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, (2018) et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung Cancer. J Clin Oncol.; 36:841–9.
View at Publisher | View at Google Scholar - Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, Bao H, Tong X, Wang X, Shao YW, (2018) et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor Osimertinib in non-small cell lung Cancer patients. Clin Cancer Res.; 24:3097–107.
View at Publisher | View at Google Scholar - Papadimitrakopoulou VA, Wu Y-L, Han J-Y, Ahn M-J, Ramalingam SS, John T, Okamoto I, Yang JC-H, Bulusu KC, Laus G, (2018) et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol.;29: mdy424.064.
View at Publisher | View at Google Scholar - Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, Lin M-C, Majem M, Shah R, Rukazenkov Y, (2018) et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol.;29: mdy424.063.
View at Publisher | View at Google Scholar - La Monica S, Madeddu D, Tiseo M, Vivo V, Galetti M, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Falco A, (2016) et al. Combination of Gefitinib and Pemetrexed prevents the acquisition of TKI resistance in NSCLC cell lines carrying EGFR-activating mutation. J Thorac Oncol.; 11:1051–63.
View at Publisher | View at Google Scholar - Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG, (2000) European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada: New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst., 92: 205-216.10.1093/jnci/92.3.205.
View at Publisher | View at Google Scholar - Tarceva®. (2017) Summary of product characteristics. In: Last updated 12;
View at Publisher | View at Google Scholar - Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, (2011) et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med.; 3:90ra59.
View at Publisher | View at Google Scholar - Cavazzoni A, Alfieri RR, Carmi C, Zuliani V, Galetti M, Fumarola C, Frazzi R, Bonelli M, Bordi F, Lodola A, (2008) et al. Dual mechanisms of action of the 5-benzylidene-hydantoin UPR1024 on lung cancer cell lines. Mol Cancer Ther.;7:361–70
View at Publisher | View at Google Scholar - Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, (2018) et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung Cancer. J Clin Oncol.; 36:841–9.
View at Publisher | View at Google Scholar - La Monica S, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Digiacomo G, Flammini L, Barocelli E, Minari R, Naldi N, (2017) et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J Exp Clin Cancer Res.; 36:174.
View at Publisher | View at Google Scholar - Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, Kuwata K, Yamaguchi H, Nishio K, Nakagawa K. (2011) Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer; 104:1594–601.
View at Publisher | View at Google Scholar - Li T, Ling YH, Goldman ID, Perez-Soler R. (2007) Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res.; 13:3413–22.
View at Publisher | View at Google Scholar - Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, Gu A, Zhong H, Wang H, Zhang X, (2017) et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer.; 141:1249–56.
View at Publisher | View at Google Scholar - Yang JC, Cheng Y, Murakami H, Yang P, He J, Nakagawa K, Kang JH, Kim J, Wnag X, Enatsu S, (2018) et al: Gefitinib with or without Pemetrexed in nonsquamous (NS) non–small cell lung Cancer (NSCLC) with EGFR mutation (Mut): final overall survival results from a randomized phase II trial. Ann Oncol, 29: viii493-viii547. 4https://doi.org/10.1093/annonc/mdy1292.
View at Publisher | View at Google Scholar - Oizumi S, Sugawara S, Minato K, Harada T, Inoue A, Fujita Y, Maemondo M, Watanabe S, Ito K, Gemma A, (2017) et al. Updated survival outcomes of NEJ005/TCOG0902, a randomized phase II study of concurrent (C) versus sequential alternating (S) gefitinib and chemotherapy in previously untreated non-small cell lung cancer (NSCLC) with sensitive epidermal growth factor receptor (EGFR) mutations. J Clin Oncol.;35.
View at Publisher | View at Google Scholar - https://www.researchsquare.com/article/rs-1106177/latest.pdf
View at Publisher | View at Google Scholar - Cui J, Zhang Y, Su D, Li T, Li Y. (2018) Efficacy of combined icotinib and pemetrexed in EGFR mutant lung adenocarcinoma cell line xenografts. Thorac Cancer.; 9:1156–65.
View at Publisher | View at Google Scholar - Feng X, Zhang Y, Li T, Li Y. (2017) Sequentially administrated of pemetrexed with icotinib/erlotinib in lung adenocarcinoma cell lines in vitro. Oncotarget.; 8:114292–9.
View at Publisher | View at Google Scholar - Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G, Peters GJ. (2008) Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol; 73:1290–300.
View at Publisher | View at Google Scholar - Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, Kris MG, Hellmann MD, Li BT, Somwar R, (2018) et al. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of mTOR as a mediator of resistance. Clin Cancer Res.; 24:3108–18.
View at Publisher | View at Google Scholar - Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas VR, (2017) et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet.; 49:1693–704.
View at Publisher | View at Google Scholar - Silvia La Monica, Roberta Minari, Daniele Cretella, Lisa Flammini, Claudia Fumarola, Mara Bonelli, Andrea Cavazzoni, Graziana Digiacomo, Maricla Galetti, Denise Madeddu, (2019) et al.Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC.Journal of Experimental & Clinical Cancer Research.; 38: 222
View at Publisher | View at Google Scholar - La Monica, S., Minari, R., Cretella, D (2019) et al. Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC. J Exp Clin Cancer Res 38, 222.
View at Publisher | View at Google Scholar - L.Gandhi,D.Rodríguez-Abreu,S.Gadgeel, (2018) et al.Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer N Engl J Med, 378, pp. 2078-2092
View at Publisher | View at Google Scholar - J.C.Soria,Y.L.Wu,K.Nakagawa, (2015) et al.Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial Lancet Oncol,16,pp.990-996
View at Publisher | View at Google Scholar - Y.Hosomi,S.Morita,S.Sugawara, (2020) etal.Gefitinib alone versus gefitinib plus chemotherapy for
View at Publisher | View at Google Scholar - non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study J Clin Oncol, 38, pp. 115-123
View at Publisher | View at Google Scholar - M.Takeda,I.Okamoto,K.Nakagawa. (2014) Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib J Thorac Oncol, 9, pp. 200-204
View at Publisher | View at Google Scholar - Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. (2008) Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada clinical trials group study BR.21. J Clin Oncol.;26(14):2350–7.
View at Publisher | View at Google Scholar - Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol.;21(7):1383–9.
View at Publisher | View at Google Scholar - Vora N, Reckamp KL. (2008) non-small cell lung cancer in the elderly: defining treatment options. Semin Oncol.;35(6):590–6.
View at Publisher | View at Google Scholar - Cioffi P, Marotta V, Fanizza C, Giglioni A, Natoli C, Petrelli F, Grappasonni I. (2013) Effectiveness and response predictive factors of erlotinib in a non-small cell lung cancer unselected European population previously treated: a retrospective, observational, multicentric study. J Oncol Pharm Pract.;19(3):246–53.
View at Publisher | View at Google Scholar - Van Meerbeeck J, Galdermans D, Bustin F, De Vos L, Lechat I, Abraham I. (2014) Survival outcomes in patients with advanced non-small cell lung cancer treated with erlotinib: expanded access programme data from Belgium (the TRUST study). Eur J Cancer Care (Engl).;23(3):370–9.
View at Publisher | View at Google Scholar - Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Larussi T, Mucilli F, Mezzetti A, (2005) et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol.;23(4):857–65.
View at Publisher | View at Google Scholar - Sugio K, Uramoto H, Ono K, Oyama T, Hanagiri T, Sugaya M, Ichiki Y, So T, Nakata S, Morita M, (2006) et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer.;94(6):896–903
View at Publisher | View at Google Scholar - Varghese AM, Sima CS, Chaft JE, Johnson ML, Riely GJ, Ladanyi M, Kris MG. (2013) Lungs don't forget: comparison of the KRAS and EGFR mutation profile and survival of collegiate smokers and never smokers with advanced lung cancers. J Thorac Oncol.;8(1):123–5.
View at Publisher | View at Google Scholar - Lee DH, Lee JS, Kim SW, Rodrigues-Pereira J, Han B, Song XQ, (2013) et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer.;49:3111–21.
View at Publisher | View at Google Scholar