Case Study | DOI: https://doi.org/10.58489/2836-8851/013
Comorbidity of impulse control disorders and major depression in Parkinson’s disease with multiple stressogenic factors: An intensive multimodal complex therapy.
- Centre for neurodegenerative diseases and Movement Disorders, Parkinson-Klinik Ortenau, Kreuzbergstr. 12-16, 77709 Wolfach, Germany.
*Corresponding Author: Ibrahim Raoua Ouedraogo
Citation: Ibrahim Raoua Ouedraogo, Jiri Koschel, David Emmans, Wolfgang H. Jost. (2023). Comorbidity of impulse control disorders and major depression in Parkinson’s disease with multiple stressogenic factors: An intensive multimodal complex therapy. Journal of Neurons and Neurological Disorders. 2(1); DOI: 10.58489/2836-8851/013
Copyright: © 2023 Ibrahim Raoua Ouedraogo, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 09 December 2023 | Accepted: 22 December 2023 | Published: 25 December 2023
Keywords: impulse control disorders, major depression, Parkinson's disease, self-efficacy, Duloxetine, Cognitive Behavior Therapy.
Abstract
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that is characterized by symptoms that impact both motor and non-motor domains [1]. The disease causes a range of movement symptoms such as balance and gait control disturbance as well as tremor. PD with comorbid major depression and impulse control disorder (ICDs) associated with various stressors not only negatively influence the overall course of disease and treatment but also lead to disability. Whereas there is a well-established treatment guideline for PD, there is no common consent protocol for an effective depression and ICDs therapy in PD Patients.
Case presentation
We present a rare and complex case of a 75-years-old patient with PD. While under treatment with Parkinson medications, the intake of pramipexole triggered the occurrence of addiction behavior. He was diagnosed with a spectrum of ICDs (pathological gambling, hypersexuality, compulsive shopping). At admission to the neurologic hospital, he was diagnosed with major depression associated with suicidal thoughts. He was dealing with a severe familial conflict and described experiencing various life stress events.
Treatment outcome
Within three weeks of stationary intensive treatment (discontinuation of pramipexole, adjustment of somatic diseases medication, antidepressant treatment, 12 sessions of CBT), most of the depression symptoms were reduced. The patient reported an enhancement of motoric symptoms and sporadic latent suicidal ideas. Beside the standard treatment of PD, this case highlights the need for an accurate intensive management of non-motor symptoms i.e., depression, ICDs in PD through the implementation of self-regulation strategies to overcome the negative thought patterns, compulsive shopping, hypersexuality and pathological gambling. The patient received simultaneously an intensive medical management for key somatic diseases and their associated neuropsychiatric disorders. He was integrated in a well-scheduled multidisciplinary therapy program to which he abided. Manifest overall health enhancements were noticeable before discharge from hospital.
Conclusion
In this study report, the implementation of a multi-modal complex therapy in a stationary treatment context was efficient enough to ease the patient’s motoric symptoms and enhance his mood state. His self-regulation and cognitive control of thoughts and action control ameliorated his ICDs. The multi-modal complex therapy helped the patient to weaken much of his childhood burden.
Introduction
The complexity of PD has attracted neuroscientists and molecular biologists who launched research aimed at understanding the puzzle beneath it. The Parkinson’s Foundation estimates that over 10 million people worldwide are living with PD [4]. Still incurable, PD is traditionally defined as a motor disease characterized by the triad of akinesia, rigidity, and tremor, whereby bradykinesia is the obligatory symptom for a secure diagnosis [1]. PD not only progressively induces movement disorders but also neuropsychiatric complications. James Parkinson [3] emphasized the manifestation of key non-motor symptoms. Over the years, neurologists worldwide have clinically documented the presence of various comorbid psychiatric disorders in PD patients.
Thus, neuropsychiatric symptoms (such as predominantly depression, anxiety, somatoform diseases, impulse control disorders, hallucinations, and sleep disorders) are frequently observed in PD. The public health approach to the occurrence of such co-morbidity occurrence in the population assumes that during ageing, the odds of depression are much higher [4, 5, 6, 7] in elderly people with multiple somatic diseases, in particular neurodegenerative disorders, cardiovascular diseases and cancer. Nonmotor symptoms such as anxiety and depression are highly prevalent in people with Parkinson’s disease, with prevalence ranging from 26% to 45% [8]. In addition, up to 40% of people with Parkinson diseases are likely to suffer from impulse control disorders due to iatrogenic effects [9]. ICDs are an aggregation of behavioral conditions that involve an inability to control impulses, sudden urges, uncontrollable desires, and their irresistible implementation that leads to risky behaviors. PD treatments may also contribute to the pathogenesis of impulse control disorders (ICDs). Attempts to understand the potential risk factors associated with ICDs pointed to personality traits, depression, concurrent use of levodopa and dopamine agonists (DAs) and difficult childhood [10]. Most neurobiological hypotheses of the genesis of depression in PD have pointed to diffuse change in neurotransmitter pathways [2]. A progressive loss of dopaminergic neurons in the substantia nigra is implicated in the pathophysiology of PD [12]. Postmortem observations revealed that loss of dopaminergic neurotransmission is thought to extend beyond the midbrain and disrupt serotonergic and noradrenergic neurotransmission in the orbitofrontal–basal ganglia–thalamic circuits [12]. The leading role of these neural systems inmood regulation explain why their disruption triggers depression and other neuropsychiatric symptoms in PD [12].
Through this image Song et al. [12] present the relevant neurotransmitter pathways and the neuronal connections between different brain regions in depression. The nucleus accumbens plays a key role as a crucial junction hub in depression-related brain regions. The authors show that two neurotransmitters, mainly GABA (gamma-aminobutyric acid) and Glu (glutamate), contribute to the signal between the nucleus accumbens and the prefrontal cortex. The neuronal circuit of two other important neurotransmitters are represented. The raphe nucleus of the brain stem delivers Serotonin, which fortifies the limbic pathway and, in the end, influences the hippocampus. The hippocampus plays a role in cognitive function. The ventral tegmentum area of the brain stem releases Dopamine, which has an impact on the entire cerebral cortex region in the brain as well as the prefrontal cortex. According to the authors the red arrows indicate the serotonin pathway, and purple arrows indicate the dopamine pathway. Blue lines show the neuronal connection between different brain regions.
Based on the research led by William James [13], Hans Selly [14], Richard S. Lazarus [15], and Aron T. Beck [16], the etiology of the major depressive disorder is believed to be multifactorial, including biological, genetic, environmental, and psychosocial factors. Also, a long-lasting exposure to various life stress events may also favour depression occurrence in elderly patients. The neuroanatomical substrate as well as the molecular mechanisms of depression in PD are being explored extensively [1]. Fortunately, in the clinical context, there is increased interest for giving equal importance to the diagnosis and treatment of motor symptoms and non-motors symptoms.
Major depression is characterised by deep sadness, mood lability, apathy, anhedonia, sleep disturbance, low self-esteem, tearfulness, emptiness or hopelessness, angry outbursts, irritability or frustration, loss of interest or pleasure in most or all normal activities and anxiety. The presence of suicidal thoughts must be considered seriously because depression and autolytic ideation are the main suicide risk factor both in elderly patients and in PD patients. Although a high prevalence of depression in PD is recorded worldwide, depression is still undiagnosed and untreated in up to 30% of patients [6]. This leads to poor quality of life and negatively affects the course of the disease. Untreated depression can result in an inability to perform activities of daily living, increased need for symptomatic PD therapy, and decreased medication adherence, which further exacerbate the disease [17].
The diagnostic of depression is clinical, following the criteria detailed in the International Classification of Diseases (ICD-10) [18] or the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, APA 2013) [19]. In elderly patients, the presence of multimorbidity and polypharmacy may complicate an accurate diagnosis of depression. It has been shown that elderly people with PD and comorbid depression respond to antidepressant treatment [20]. A reduction in depression symptoms and the strengthening of coping capabilities against addiction pressure, i.e., impulse control behaviours, is possible through a multidisciplinary approach. This supposes a combination of antidepressant drugs, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SSNRIs) and psychotherapeutic approaches (cognitive-behavioural psychotherapy). The concomitant treatment with antidepressants and CBT has shown therapeutic efficacy [21].
The relevance of properly treating depression is evidenced by numerous scientific publications [7, 63], which increase awareness for the need to simultaneously manage motor and non-motor symptoms in PD. While depression is common in PD, depression associated with a range of impulse control disorders i.e., pathological gambling, hypersexuality, compulsive shopping, suicidal ideas in a context of manifold psychosocial stressors in Parkinson Patients is sporadic and rarely reported. Some cases presenting in ambulatory settings are either underdiagnosed or not given treatment priority. An efficient management of such complex cases remains a challenge as it requires hospitalisation in a special neurological unit that offers a multidisciplinary treatment option. We report this case of PD with multiple neuropsychiatric symptoms (depression-impulse control disorders, various psychological burdens), which received priority for multimodal therapy i.e., an intensive simultaneous medical treatment of somatic diseases combined with neuropsychiatric and psychotherapeutic treatments (CBT). The multimodal treatment that lasted three weeks also included physiotherapy, occupational therapy, and social welfare/work counselling.
The first aim of this study is to describe such a complex case that required a multimodal approach, i.e., diagnosis, treatment, and a plan for a sustained psychological support during a stationary stay in hospital. Such a multimodal approach may alleviate current motoric symptoms in PD and at the same time reduce depression and its corollary neuropsychiatric symptoms. The second aim is to raise the attention of health professionals about such an uncommon complex Parkinson’s disease manifestation with multimorbid neuropsychiatric symptoms. We hope that this clinical study will contribute to the enhancement of the treatment protocol of similar cases encounter elsewhere.
Case Presentation
The clinical case describes a male PD patient (Timbo) with a diagnosis of ICDs (pathological gambling, hypersexuality, and compulsive shopping) and major depression. His inability to control his compulsive gambling, his increased craving for sexual behavior, and compulsive shopping provoked a severe familial conflict. The patient encountered a wide range of psychosocial burdens. His complicated relationship during childhood to his father (who did not want to recognise him as his son) was one of the most emotional painful psychological distresses in his life. As he said with tears in his eyes: “My father did not want his family and other people to know that I exist. I have been fighting permanently for everything in my life”. According to his own statements, he had been dealing with depression two years prior to the diagnosis of PD.
Diagnosis
Parkinson’s Disease of akinesia rigor type, G20.10, Hoehn and Yahr stage III (diagnosed in 2015).
Impulse control disorders, F63.8, (Hypersexuality, pathological gambling, compulsive shopping) under treatment with pramipexole.
Major depression, F32.2, with severe anxiety and genuine suicidal ideas.
75 years old, with a diagnosis of PD, Hoehn and Yahr stage III (diagnosed in 2015). He was suffering from depression two years prior to the diagnosis of PD, but a thorough assessment of his mood state did not occur during preliminary examination. As the symptoms of PD appeared in 2015, one of his friend’s raised attention to it and suggested that he seek professional help by a neurologist. Because he found it shameful to be diagnosed with PD, he did not go to see the neurologist. He decided to see a neurologist only six months later. Under treatment with pramipexole, impulse control disorder appeared, in particular pathological gambling, hypersexuality and compulsive shopping. These impulse control disorders along with their psychosocial impacts (uncontrolled extravagant money spending and behaviour risk due to increased libido and sexual behaviour) led to a serious familial conflict. His two sons and even his friends stopped talking to him and made it clear that he needed stationary psychiatric and psychotherapeutic treatment. He was upset, felt isolated and guilty.
The diagnosis of major depression was first made in 2022 during a neuropsychological examination in our hospital. He admitted having with suicidal and anxiety thoughts and asked for a treatment for depression alongside his current treatment for Parkinson symptoms.
The patient is 75-years-old, widow and retired manager of an oil company. His first marriage lasted 12 years. He then divorced. He described his divorce as painful. He has three children from his first marriage (three sons). Two years later, he was engaged in a relationship. He married again but unfortunately his second wife died ten years later. One of his sons who joined the military was deployed in Afghanistan where he died while on duty. The death of his son made him very sad and helpless. He has four grandchildren. He denied alcohol abuse.
Personal history
The patient is the only child from his mother’s side of the family. He has three older half siblings. His was born after his mother became pregnant following an affair with a famous married man. His biological father, a mayor, was legally married and had three children with his spouse. For this reason, he did not want the case to be made public. He even did not want to meet him during his childhood. His biological father did not change his stance even when Tom turned adult. This led him to depict his childhood as poisoned, characterised by a feeling of injustice, psychological pain, a feeling of rejection, the conviction of not being desired and not loved. He was bullied at school. He said: “My childhood was appalling; it was full of tears and shame. I was fainting und crying silently.”
Later his mother wed another man who was an executive director of a multinational company. The relationship with his stepfather was mixed. He claimed he loved him but did not have time for him. There were not many contacts, any emotional and any affective relationship with his biological father. Thus, there was no effective emotional relationship with his stepfather. Because of the lack of social support, he admitted that he was left alone dealing with anxiety, negative self-image, feelings of inferiority, negative thoughts, and negative mental pattern thoughts. He had to fight to be seen and to be eulogized by his stepfather and at the time he was fighting to be recognised by his biological father. The school years were difficult because he was bullied. Even back then, his mood state was instable, and he tended to avoid social contact. After he took his A Level exam, he went to study economical sciences. He graduated in finance and organisational management and earned a diploma.
Methodology
After the admission formalities, the patient was thoroughly examined by a neurologist. Further detailed examinations and tests were also performed.
Medical Examination
His blood pressure was 140/90 mm Hg, and his pulse is regular at 110 beats per minute. The rest of the physical examination did not reveal any abnormality.
Laboratory: The laboratory examinations showed an HbA1c of 6.3%. Vitamin B12 was reduced at 293 ng/l. Otherwise, the results were normal for the remaining laboratory parameters.
Pupillographic sleepiness test: PUI 3.2 mm/min, MRS 95, thus no increased daytime sleepiness.
Residual urine sonography T1: 176 ml residual urine.
Control T2: 208 ml residual urine.
Control T3: 172 ml residual urine.
Posturography: Significant impairment of stance stability.
Circulatory function test: Normal resting blood pressure, drop in systolic blood pressure of 23 mmHg, thus orthostatic hypotension is present.
Odor test with Sniffin`Sticks: Anosmia, 4 out of 12 offered odorants were correctly recognized.
Neurography of the right tibial nerve: normal distal motor latency. The patient did not tolerate further examination.
Neurography of the right median nerve: normal latency, amplitude and conduction velocity.
A cMRI of the skull was performed externally on 14.07.2022. Otherwise, regular intracerebral findings. The results revealed no increased cerebral atrophy. No space-occupying process. The results revealed no increased brain atrophy. The image is unfortunately not accessible but will be inserted as soon as available.
ECG: Sinus rhythm/sinus frequency, HF 64/min, QTc-Zeit 427 ms. The results revealed no abnormality.
EEG: 8/s Alpha-EEG. No potentials specific to epilepsy. No focal findings. Two sequences with dysrhythmic higher-tension theta waves for 3 to 4 s. No reaction in the Berger trial. The results revealed no abnormality.
Neuropsychological Examination
During the first examination, he reported that he had been suffering from mood lability since the beginning of the year 2013. He indicated being under inner restlessness, anxiety, and psychological strain. He described a negative self-image and fear of breakdown. Also, he acknowledged dealing with suicidal ideas, low self-esteem, and permanent confrontation with negative thought patterns. He mentioned that his addiction behavior led to a serious conflict with his children and friends. He needed help “I cannot go back home without being able to cope efficiently with these psychological stressors. I no longer have any joy in life.”
Montreal Cognitive Assessment (MoCA) [23]
He took part in neuropsychological assessment, which did not reveal any cognitive abnormalities in the MoCA, 28/30.
Frontal Assessment Battery Dubois et al. [24]
The Frontal Assessment Battery (FAB) is a brief battery of six neuropsychological tasks designed to assess frontal lobe function at bedside.
The FAB, at a score of 18/18, did not reveal a frontal brain syndrome.
BDI-II (Beck’s Depressions-scale) [25]
During the first examination, he reached a total score of 33 points, which indicated that he had a major depressive episode (F32.2). At the second examination, he reached a total of 19 points, which points to a mild depressive episode (F32.0). The patient received treatment with duloxetine combined with an intensive supportive CBT (12 individual sessions).
General Self-Efficacy Scale (GSE) [26] and self-regulation training
During the first examination, the patient scored 10 points, which falls within the range of a low self-efficacy. During the second examination, he scored 25 points, within the range of a mild self-efficacy.
Hamilton-Anxiety Scale/ HAMA [27]
During the first examination, the patient scored 40 points, which falls within the range of a severely elevated value of anxiety. During the second examination, the patient scored 20 points, within the range of a mild elevated value of anxiety. The permanent and pronounced anxiety as well as the hopelessness, the mood swings and the depression were indicative of a major depression associated with severe anxiety.
SCL-90-R: Derogatis [28]
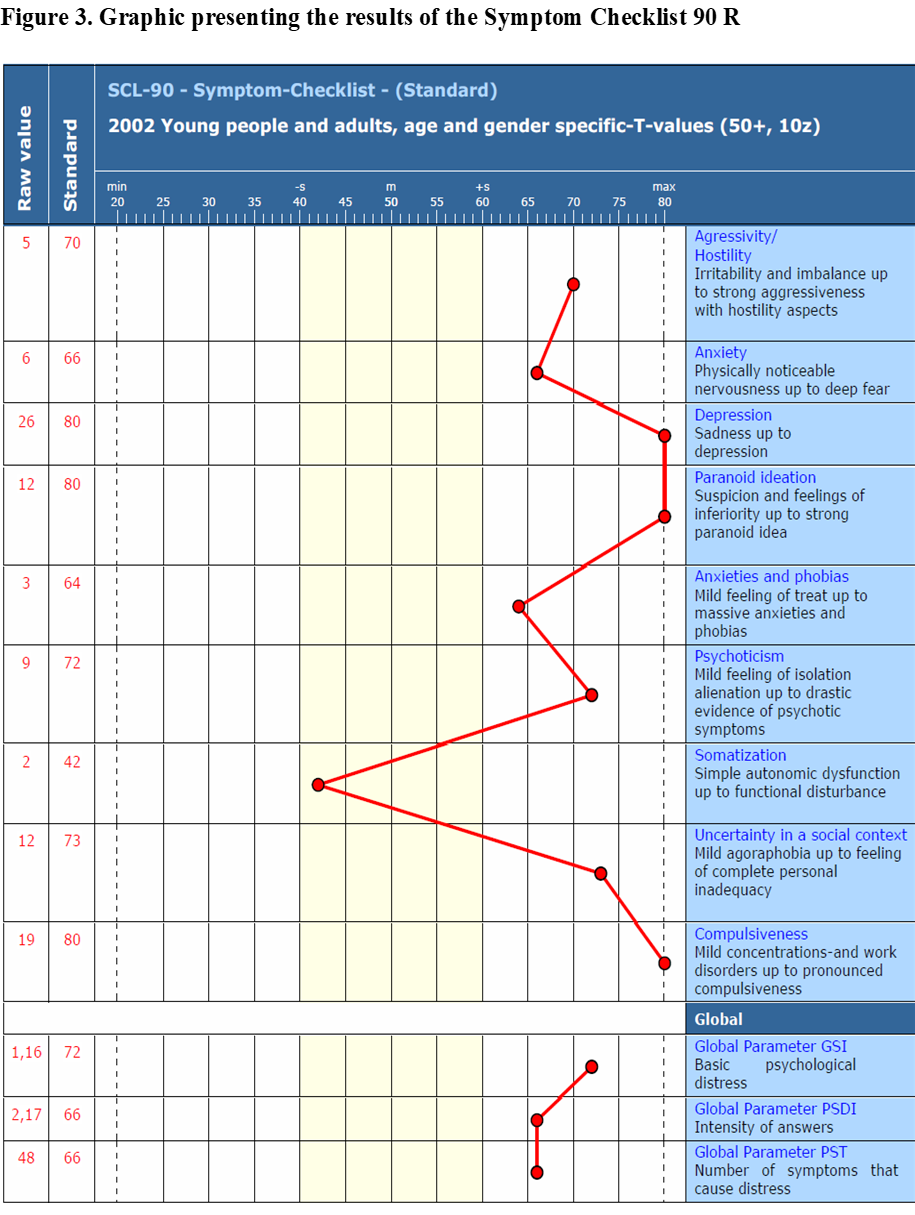
During the admission test, six subscales produced T values that were above the critical threshold of 60: Depressiveness - 80, Aggressiveness/hostility - 70, Anxiety - 66, Paranoid thinking - 80, Phobic anxiety - 64, Psychoticism - 72, Insecurity in social contact - 73, Compulsiveness - 80. Of the global indicators, the GSI (general psychological distress) - 72 and PST (number of symptoms of distress). 66 indicates elevated scores.
Berg Balance Scale [29]
During the first examination, the patient scored 42 points, which indicates an independence in mobility thought a minor deficit (-14 points) in balance and gait control. During the second examination, the patient scored 56 points, which indicate an independence in mobility and an enhancement in balance and gait control (an increase of 14 points).
Medical history
Development of the disease: The Parkinson's disease began in 2013 with reduced step length and a micrography. In 2015, a clinical diagnosis of Parkinson's syndrome of the akinetic-rigid type was made in a specialized neurological hospital in Germany. The patient was then treated with pramipexole base (Sifrol®) 0.52 mg twice daily. In the subsequent course of the disease, the medication was extended to include Stalevo®. After the pramipexole (Sifrol®) caused side effects in the form of impulse control disorders (the patient described a tendency towards increased sexuality, gambling and compulsive shopping), it was discontinued and replaced by the MAO-B inhibitor rasagiline (Azilect®).
Actual complaints: Small-step gait pattern, right-sided rigor and tremor of the upper extremity, intermittent dyskinesias during the day, postural instability. In addition, the patient described his mood state as unstable and reported that he often felt depressed.
Interim medical history: He reported that he had noticed a loss of efficacy in the medication, with an increase in off-times, difficulty speaking and freezing.
Current social situation: He lives alone. He has a long-term care level of 3. The patient is independent when in an on-phase but is dependent on help when in an off-phase.
Current complaints: The main symptoms are motor fluctuations (connected with gaps in medication effectiveness) in particular pronounced off-times (increased after discontinuation of entacapone) with freezing, increase in cognitive disorders and speech disorders. He notices cognitive impairments, which he can, however, compensate for very well intellectually; the patient is still able to make decisions.
Vegetative: Appetite good, weight constant recently. Micturition unremarkable, nocturia once or twice. Rarely constipation. Poor sleep with difficulty falling asleep and staying asleep as well as occasional nightmares with violent movements. He fell out of bed once in his sleep about 6 months ago. No alcohol or nicotine abuse.
General clinical findings: 75-years-old patient with good AC, normal EC, no cyanosis, no edema, skin and visible mucous membranes well perfused. Cor: pure, rhythmic. No pathological heart sounds. Pulmo: bds. vesicular breath sounds. Abdomen soft, no resistance, no tenderness, liver palpable below the costal arch. Spleen not palpable. Renal bed free. The large and small joints are freely movable on all sides, peripheral pulses palpable.
Neurological: Right-sided rigor and tremor, limited arm pendulum on the right. Small to medium-stepping and bound gait, difficult body position changes, postural instability, cervical dystonia with lateral shift (laterocollis to the left, laterocaput to the right). Cranial nerves: NAP free. Eyelid fissure unremarkable, infrequent blinking, no eye spasms, pupils moderately dilated, isocoric with prompt light reaction, no nystagmus. Anamnestically reduced sense of smell. Visual acuity corrected with glasses. Hypomimia. Speech and articulation slightly slowed. The muscle reflexes of the upper and lower extremities can be triggered discretely to midline on the same side. No positive pyramidal tract signs. Arm and leg hold test unremarkable. Sensorium for esthesia and algesia orienting intact. Vibration sensation bimalleolar 4/8. Position sense of the legs intact. Coordination: FNV and KHV inconspicuous. Dysbradydiadochokinesia accentuated on the right. Limited fine movement of both hands. Retropulsion without compensation in the pull test.
Psychopathological findings on admission
He was alert and fully orientated towards all qualities. He appeared overly careful and mistrustful when making a first contact. Progressively, an affective rapport was established. No higher-grade disorders of cognitive functions and perception were noticeable. Attention, concentration and memory were clearly normal, but he reported subjective memory impairment. His psychomotor functions were tense, and his mood was severely depressed with a reduced capacity for affective empathy. The goal-orientated behavior was considerably reduced. There was a pronounced feeling of desperation and helplessness as well as a considerable fear of isolation. The patient’s self-efficacy and self-esteem were low. There was no evidence of delusions, ego disorders or hallucinations. Although he reported dealing with suicidal ideas, there was no acute suicidal tendency and no plan to implement it. He had no intention to commit suicide in the hospital. Insight into the illness and motivation for a stationary treatment were present.
Genesis of psychiatric symptoms
The psychological vulnerabilities involved his childhood difficulties, (not recognised by his biological father, feeling of rejection, bullying), divorce, becoming a widow, and the death of his son in Afghanistan. It is likely that mild to moderate depression has plagued him since the death of his wife in 2005 and his son in 2012. These stressful life events may have worsened his mood instability after he was diagnosed with PD in 2015. It was only two years prior to his diagnosis with PD that he became conscious of it. He later faced fierce criticisms and rejection because of his ICDs induced by the
medication with pramipexole. Since then, his depression symptoms have worsened, and he acknowledged having suicidal thoughts and feelings of guilty.
Behavioral analysis and the genesis of depression:
Many years of past life stress as well as many stressors from life events and frustrations must be considered as vulnerability factors for the development of his depressive disorder. The triggers for the depressive episodes in the past included diverse stressful life events such as the persistent childhood burden, the absence of a father, the self-image disorder, the occurrence of excessive life events stressors (the death of his wife and his son). His work as a company manager was stressful for him. In his childhood, the patient felt helpless and powerless. He has experienced bullying. The causes for the current depressive episode are probably the conflicts with his children and friends who rejected and isolated him because of the psychosocial adverse effects of his ICDs. He permanently sought recognition from his father. Unfortunately, he continued to experience rejection. Feelings of rejection, isolation, fear of family breakdown, lack of social support, lack of coping skills, massive stress in childhood, self-image disorder, frustration (the deaths of loved ones), insecurity, negative self-esteem, low self-efficacy led to severe depressive symptoms.
Question: What are the treatment recommendations? Due to the complex association of multimorbid neuropsychiatric symptoms alongside his motoric fluctuations and the urgent need for medication adjustment, the neurologist decided on a multi-modal complex therapy that last three weeks.
Multi-modal complex therapy and treatment goals:
- Discontinuation of pramipexole
- Adjustment of Parkinson medicines and treatment of key motoric symptoms (Stalevo, Xadago)
- Medical treatment of depression (Duloxetine)
- Coping with depression
- Self-regulation, coping and cognitive control of addiction pressure (ICDs)
- Treatment of motoric symptoms (physiotherapy, speech therapy, occupational therapy).
Senior neurologists implemented and monitored the discontinuation of pramipexole which has been thoroughly replaced by L-Dopa therapy. The psychological interventions were carried out by a senior psychologist with extensive experience in acute psychiatric hospital treatment for cognitive behavioral psychotherapy (CBT) and geriatric psychiatry as well. The development of cognitive and behavioral coping strategies was essential for the therapy process. We included the corollary effects of ICDs (shame, fear, rejection, and familial conflicts, suicidal ideations) in the CBT. Intensive psychological intervention: 12 sessions of CBT, 6 sessions of relaxation, 6 sessions of supportive group therapy.
Table 1: sessions of individual psychological talks based on CBT.
| Sessions | Psychological Support: CBT-Self-Regulation |
| 1st Session | Risk perception and motivation for behaviour change (counteract negative thoughts, coping with grief, enhancing self-image, addiction consequences, conflicts with relatives) |
| 2nd, 3rd, 4th. Session | Goal setting and plan for behaviour change. Goal implementation |
| 5th, 6th, 7th, 8th Session | Self-esteem enhancement, autoregulation coping with cognitive dissonance, depression-addiction behaviours |
| 8th ,9th Session | Coping with past negative stress, training for self-efficacy enhancement, mastery and control
|
| 10th, 11th Session | Self-efficacy enhancement-implementation Regulation of cognitive dissonance |
| 12th Session | Final assessment and plan for a stationary treatment in an acute psychiatric hospital Self-esteem enhancement, coping with the negative. Thought-depression-impulse control disorders |
Pramipexole was discontinued and Levodopa was initiated. This was associated with good effects on motor fluctuations as shown by a decrease in the patient’s total UPDRS [65] score from 67 before Levodopa to 43 during Stalevo (100/25/200mg) and Xadago (100mg). Duloxetine (60mg) was administered to alleviate symptoms and suicidal ideas. The individual sessions were characterized by a high degree of ambivalence and tension regarding the further harmony of the family because of the impulse control disorder. The patient showed pronounced deficits in terms of self-image, self-esteem, self-efficacy, and autoregulation. He suffered from intrapsychic conflicts, irrational thought patterns and a loss of control over family conflicts. Through the strategy for changing his behavior, which is based on the method of self-regulation and active cognitive behavioral change, he was able to activate his perception of risks (addiction pressure, illusions, cognitive distortion) and his outcome expectancy “expectation of action results” (pros and cons) to gain a greater degree of self-confidence and self-esteem.
After five video conferences with the two children (interactive conversations, i.e., the two children, the patient, and the therapist), he received a letter from the children. In the letter, the children expressed their willingness to restore family harmony if he took advantage of an inpatient acute psychiatric treatment to put his addictive behavior under control. Receiving this letter made him happy and encouraged him not to give up. A coping option for self-regulation strategies, cognitive control of thoughts and actions was developed. Another focus of the treatment was the promotion of positive self-image and mood regulation through cognitive coping strategies. The underlying feelings of shame and rejection were also addressed. The patient was able to shift his negative self-esteem into a positive one whereby enhancing his self-efficacy. The adoption of healthy behavior through risk appraisal, self-regulation as well as coping with the fears of the family breakdown and his isolation from friends were very important for the patient. In a mental spiral of negative and depressive thoughts, he kept fantasizing about how bad it would be if he could no longer see his children and grandchildren, thereby increasing his fears. The video conference helped to reduce his anxiety. In the progressive relaxation group, he was able to learn how to influence his own body and reduce his stress levels more effectively. He showed an improvement in the Unified Parkinson’s Disease Rating Scale (UPDRS) at discharge. The Berg Balance Scale at discharge unveiled an improvement (42 versus 56). As for the motoric functions, the patients reported fewer motor fluctuations, i.e., rare off-phase and only cases of isolated freezing. The individual training in physiotherapy reduced his postural instability, enhanced his functional mobility and he reported less dyskinesia. At the second assessment of the GSE, we recorded an enhancement in Self-efficacy (mild versus low). The Beck-II depression scale and the Hamilton anxiety scale also yielded significant improvement in depression and anxiety at the second time point assessment.
Table 2: Neurological examinations, neuropsychological and physiological assessments
| Assessments | Admission score | Score at Discharge |
| MoCA | 28 | stable |
| FAB | 18 | stable |
| Beck-II depression scale | 33; major depression | 19; mild depression |
| Hamilton anxiety scale | 52; severe anxiety | 17; mild anxiety |
| UPDRS | 67 | 43; improved |
| Berg Balance Scale | 42 | 56; improved |
| Suicidal Ideation | Manifest, clinical evaluation | Latent/ improved |
| Self-efficacy scale | 10; low Self-efficacy | 25; improved |
Treatment results
He was able to achieve a relative stabilization during his inpatient stay. The initial depressive disorder partially remitted, but the test at discharge shows the persistence of a pronounced depressive syndrome. We were able to discharge the patient is in a relatively better psychophysical condition. We strongly recommended further inpatient treatment in an acute psychiatric hospital with additional therapy to cope with addiction pressure. We considered this essential to stabilize the therapeutic successes achieved here. Due to the cessation of pramipexole and the adjustment of the key medications and the multi-modal complex therapy, a significant improvement in motor and non-motor symptoms was noticed before discharge from hospital. He continues to require outpatient therapeutic care. If necessary, an extension of the ambulatory psychotherapeutic (CBT) sessions should be made.
Recommendations
1. Neuropsychological examination, Psychiatry and Psychotherapy
An inpatient treatment for depression and addiction behaviors was recommended.
2. Somatic recommendations
Regular ambulatory neurological examinations. Further monitoring of cardiovascular parameters and ECG as well as routine laboratory parameters during drug therapy.
Discussion and Conclusion
In this study on an adult male patient with PD and multimorbid neuropsychiatric symptoms, we present a case of successful treatment with Parkinson medication adjustment, pharmacological treatment of depression combined with an intensive psychological intervention.
Throughout this study report, the following areas of deliberation are raised:
- Role of pramipexole treatment in ICDs.
- Multifactorial etiology of the depression.
- Diagnostic certainty and treatment of depression.
- Efficacy of multi-modal therapy in stationary context.
- The beneficial effect of psychological intervention based on CBT and the role of social support in the patient’s care.
- Reflection on inclusion and exclusion criteria for dopamine agonist treatment in PD.
1. Pramipexole and impulse control disorders in PD.
Impulse control disorder (ICDs: including pathological gambling, hypersexuality, and compulsive shopping) has been linked to antiparkinsonian medication, especially dopamine agonists. Dopamine agonists are the most frequent drugs associated with ICDs [30]). Certain subpopulations such as younger patients [31] do have increased risk. PD itself does not seem to confer an increased risk for development of ICDs because newly diagnosed but still untreated patients with PD do not have an increased risk of developing ICDs when compared to controls [32]. Dopamine agonists elevate the risk of ICDs not only in PD, but also in the restless legs syndrome [33], thus making ICDs mainly a drug-related side effect. The mechanism of ICD is not completely clear, but it seems that ICDs is the result of an activation of dopamine receptors, mostly D3 in the ventral striatum [34]. PD patients treated with dopamine agonists that have preferential affinity for D3 such as pramipexole or ropinirole are much more prone to develop ICDs than dopamine agonists with lower affinity for D3 receptors such as rotigotine or apomorphine [34]. A genetic component is probably present, especially in young patients. Environment and lifestyle may also play a role: Those patients engaged in physical, social, and artistic activities are probably less likely to develop ICDs compared to those patients with poor physical activity, living in isolated environments [35]. In the case of our patient, it was necessary to withdraw pramipexole because of the negative impact caused by the ICDs. We restored the deterioration of motor symptoms by the increase of levodopa. When reducing pramipexole, we had to be aware of a possible deterioration of the depression, because pramipexole also has shown antidepressant efficacy as an augmentation strategy for unipolar and bipolar depression due to its D3 affinity [36].
2.CBT intervention strategies for the management of ICDs.
PD with an array of ICDs (libido increase, compulsive shopping, pathological gambling) not only led to a conflict with close relatives and further psychosocial burden but also caused difficulties to general health and was disastrous for the patient himself. In our patient’s case, his addictive behavior disrupted his relationship to two sons and his friends, who were suffering from mental stress due to his risky behaviors (increased libido and urge in sexual behavior, lavish spending of money). To get a significant remission of ICDs, we coupled discontinuation of pramipexole with CBT focused on self-regulation, self-efficacy training and cognitive control of compulsive behavior i.e., autoregulation of addiction pressure and compulsive libido satisfaction. We created a systemic therapy through the video conferences.
The role of social support in the addiction coping process is important [9, 37]. In a longitudinal study, it was shown that ICDs resolved after 1 year in about 50% of the patients who stopped dopamine agonists and continued to improve [38]. In the case of our patient, the increase of levodopa dosage helped avoid worsening in motor symptoms [39]. However, treatment is still challenging, as patients may experience a dopamine agonist withdrawal syndrome [39, 40, 41], which can lead to relapse. To help maintain his coping capabilities against ICDs and solve the conflicts with his children, he accepted to go for an inpatient intensive psychiatric treatment after discharge. Some clinical pointers showed that the patient was probably at high risk for developing ICDs under pramipexole’s treatment. Such clinical parameters could help to filter the candidates for a dopamine agonist (DAs) treatment.
3. Multifactorial genesis of depression and treatment outcome in major depression: Effect of a combined antidepressant treatment and CBT.
The case of our Patient matches with the multifactorial genesis of depression in PD. Indeed, many vulnerable factors were present during his development. He himself admitted that he probably already had depression prior to the diagnosis of PD. A disturbance in key neurotransmitters (serotonin, dopamine) involved in mood regulation occur quite often in PD prior to the diagnosis [42, 2]. Depression and anxiety are thought to develop before dopamine cell loss in the substantia nigra pars compacta, and therefore before motor symptoms are evident [43]. This mixture of mood dysregulation is thought to be linked to brainstem monoamine pathology affecting the locus coeruleus and the dorsal raphe nucleus at the early stages of the disease [44, 45]. In fact, increased anxiety associated with depression manifesting later during PD probably reflects alterations in multiple neurotransmitter pathways [44]. The monoamines (such as dopamine, noradrenaline, and serotonin) are specifically affected by these changes [45]. Now, there is increasing evidence from preclinical and clinical studies for the involvement of glutamatergic and GABAergic transmission in depression and anxiety in PD [42, 43]. Dopamine interactions with the limbic system are probably involved in stress and depression [46, 9]. The reduction of catecholaminergic innervation in depressed Parkinson’s disease patients occurs in regions thought to comprise the emotional circuits of the brain [42]. The amygdala, mediodorsal thalamus, ventral striatum and CingA belong to the limbic system and have been implicated as dysfunctional regions in mood disorders [47, 48]. The amygdala is a key structure for emotional processing in humans [49]. Functional abnormalities in the amygdala correlate with severity of endogenous depression [47, 50], and the amygdala mediates fear processing and anxiety [49]. The amygdala connects with locus coeruleus and receives a noradrenergic and dopaminergic innervation [ [51, 52], which is reduced in PD [53]. In addition, it has been reported in a post-mortem study that PD patients have up to a 20% reduction of amygdala volume and that this structure contains Lewy bodies [54].
Through this image Prange et al. [55] depict the functional and structural dysfunctions related to depression in PD. As they mentioned it, red: striatum; light green: prefrontal cortex and insula; green: brainstem; light blue: cingulate cortex; blue: thalamus; violet: amygdala and hippocampus). ACC anterior cingulate cortex, sgACC subgenual ACC, Amyg amygdala, CN caudate nucleus, GP globus pallidus, GR gyrus rectus, Hipp hippocampus, HypoT hypothalamus, Ins insula, LC locus coeruleus, MTG medial temporal gyrus, OFC orbito-frontal cortex, PCC posterior cingulate cortex, PFC prefrontal cortex, Put putamen, VS ventral striatum.
The patient received a pharmacological treatment with duloxetine (SNRIs), which was combined with intensive supportive individual talks based on CBT. The choice of SNRIs is based on clinically proven efficiency. SNRIs appear to be the safest medication for the treatment of depression in PD, resulting in the fewest side effects. In a series of individual talks, he received the opportunity to reflect his past and actual psychological difficulties. We offered him a range of coping and self-regulation strategies aimed at decreasing negative thoughts, suicidal ideation, addiction pressure and behaviour change. The combined treatment yielded a significant change in depression, a reduction in depressive symptoms was clinically manifest, and a minor to moderate depression was recorded at the second test (Beck II depression scale: 19 points). The suicidal ideations were sparse and an enhancement in functional ability and mobility were manifest. He still had depression before release but given the complex combination of ICDs, depression and family strain, we did not expect a full remission of depression within three weeks.
4.Treatment with Serotonin norepinephrine reuptake inhibitor (SSRI)
Efficiency of antidepressants in PD depression was confirmed in the three largest randomized clinical trials with non-demented patients with PD and major depressive disorder, respectively [56, 57]. However, in PD, SNRI are the safest medication with high efficacy for depression as well [58]. SSRIs are not only effective for treating PD depression but have also been demonstrated to favor positive effects on motor ability and activities of daily living. Poor adherence to the antidepressant regimen can be a symptom of depression and Parkinson's.
Among other non-pharmacological treatment strategies, we refer to the implementation of physical activity (Nordic Walking, Tai Chi exercises, sport) [59] which have a beneficial effect in treating depression in PD. In addition, aerobic training exercise, Qiqong, dance, and yoga also have a beneficial effect on depression and motor function in PD. Indeed, physical exercise was demonstrated to enhance mesolimbic functioning and increase dopaminergic release in the caudate nucleus [60] and may promote the anti-inflammatory response [61, 62].
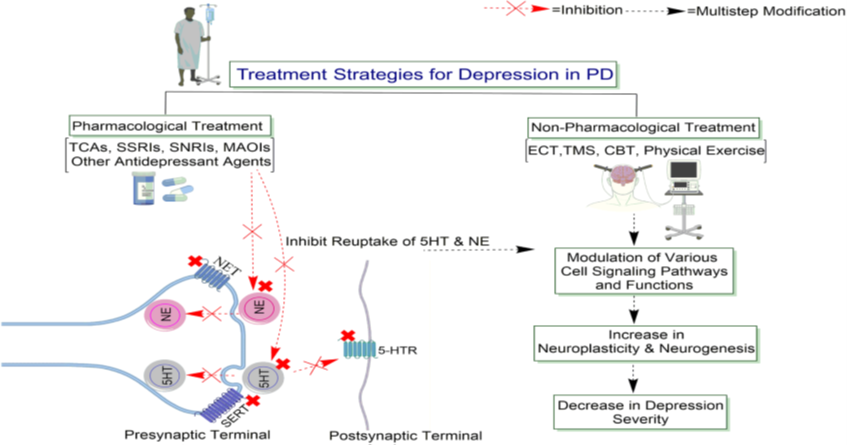
In this image Ahmad et al. [63] review the antidepressant treatment of depression in PD. They show that the antidepressant and non-pharmacological treatments for depressed PD patients increase noradrenergic or serotonergic neurotransmission by blocking the NE or 5HT transporter at presynaptic terminals (NET, SERT), producing long-lasting changes in monoaminergic neurotransmission. The authors also emphasize that the inhibition of the reuptake of 5HT and NE may enhance the neurotransmission, presumably by prolonging the dwell-time of the transmitters in the synapse. It is interesting to notice that the sustained signaling via NE or 5HT increases the expression of specific downstream cell signaling pathways and functions, thereby increasing neuroplasticity and neurogenesis. Abbreviations: 5HT, 5-hydroxytryptamine; 5-HTR, 5-hydroxytryptamine receptor; CBT, Cognitive-behavioral therapy; ECT, Electroconvulsive therapy; MAOIs, Monoamine oxidase inhibitors; NE, norepinephrine; NET, norepinephrine transporter; SSRIs, Selective serotonin reuptake inhibitors; SNRIs, Serotonin-norepinephrine reuptake inhibitors; SERT, serotonin transporter; TCAs, Tricyclic Antidepressants; TMS, Transcranial magnetic stimulation.
Conclusion
In this study report, we dealt with a complex combination of multiple neuropsychiatric symptoms after severe stressors and PD. We presented a case study of successful treatment based on a multi-modal complex therapy in a stationary context. Depression belongs to one of the prominent neuropsychiatric symptoms in PD. Even if mild with comorbid ICDs, it has a potential devastating effect and therefore need a proper treatment. The withdrawal of pramipexole, the adjustment of levodopa, the antidepressant treatment of depression and the concomitant intensive psychological intervention have proven an efficacious enhancement of motoric symptoms and depression. As Weintraub and Claasen [64] suggested, insight into patient clinical and neurobiological parameters may guide a precise medical approach and decrease the incidence of ICDs in PD by determining those at high risk (e.g., genetics, depression, dopamine transporter neuroimaging, difficult childhood, impulsive personality traits, personal or family history of substance abuse, affective bipolar disorder). Because of the potential risk of relapse of compulsive behaviour and depression, patients with PD and comorbid ICDs and depression should receive an ambulatory psychotherapeutic treatment. The risk factors for relapse are magnified by the persistent intra-psychic conflicts, psychosocial problems, interpersonal problems as well as further psychiatric disorders.
Declarations
Data Availability
The data analyzed in this study is subject to the following restrictions: identifying confidential patient data cannot be shared. The data are not publicly available due to confidentiality and anonymity.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. As a single case report, ethical review and approval were not applicable.
Informed Ethic Consent Statement
Informed consent, following the recommendation of the Galician. Written informed consent was obtained from the patient for anonymous publication of this article.
Authors Contributions
All authors participated substantially in the writing and editing of the final manuscript.
Acknowledgments
We would like to thank the participant of this study for his cooperation and his contribution to a real-world clinical research scenario dealing with complex treatment issues in PD. Special thanks to Ms Tanja Fiesel, Ms Beate Becht and Ms Petra Schuler who managed the administrative and diagnostic procedures.
Conflicts of Interest
The authors have no conflict of interest to declare.
Sources of Funding
This research received no funding.
References
- Jost, W. H., & Reichmann, H. (2019). Time for a new definition of Parkinson’s disease. Journal of Neural Transmission, 126(7), 801-801.
View at Publisher | View at Google Scholar - Santiago, J.A., Bottero, V., & Potashkin, J. A. (2017). Biological and clinical implications of comorbidities in Parkinson’s disease. Frontiers in aging neuroscience, 9, 394.
View at Publisher | View at Google Scholar - Parkinson, J. (2002). An essay on the shaking palsy. The Journal of neuropsychiatry and clinical neurosciences, 14(2), 223-236.
View at Publisher | View at Google Scholar - McDonald, R. B., & Ruhe, R. C. (2011). Aging and longevity: why knowing the difference is important to nutrition research. Nutrients, 3(3), 274-282.
View at Publisher | View at Google Scholar - Steffen, A., Nübel, J., Jacobi, F., Bätzing, J., & Holstiege, J. (2020). Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC psychiatry, 20(1), 1-15.
View at Publisher | View at Google Scholar - Berk, M., Köhler‐Forsberg, O., Turner, M., Penninx, B. W., Wrobel, A., Firth, J., ... & Marx, W. (2023). Comorbidity between major depressive disorder and physical diseases: a comprehensive review of epidemiology, mechanisms and management. World Psychiatry, 22(3), 366-387.
View at Publisher | View at Google Scholar - Ibrahim Raoua Ouedraogo (2023). Influence of baseline comorbid diseases on major depression and the effect of intensive medical treatment on functional mobility in depressed patients compared with those without depression. Psychiatry and Psychological Disorders (PPD) 2 (2).
View at Publisher | View at Google Scholar - Fernandes, M., Pierantozzi, M., Stefani, A., Cattaneo, C., Bonizzoni, E. A., Cerroni, R., ... & Liguori, C. (2021). Frequency of non-motor symptoms in Parkinson's patients with motor fluctuations. Frontiers in Neurology, 12, 678373.
View at Publisher | View at Google Scholar - Zhang, J. F., Wang, X. X., Feng, Y., Fekete, R., Jankovic, J., & Wu, Y. C. (2021). Impulse control disorders in Parkinson's disease: epidemiology, pathogenesis and therapeutic strategies. Frontiers in Psychiatry, 12, 635494.
View at Publisher | View at Google Scholar - García-Rubio, M. I., Otero-Cerdeira, M. E., Toledo-Lozano, C. G., Alcaraz-Estrada, S. L., Suárez-Cuenca, J. A., Coral-Vázquez, R. M., ... & García, S. (2021, September). Analysis of impulse control disorders (ICDs) and factors associated with their development in a Parkinson’s disease population. In Healthcare (Vol. 9, No. 10, p. 1263). MDPI.
View at Publisher | View at Google Scholar - Seppi, K., Ray Chaudhuri, K., Coelho, M., Fox, S. H., Katzenschlager, R., Perez Lloret, S., ... & Djamshidian‐Tehrani, A. (2019). Update on treatments for nonmotor symptoms of Parkinson's disease—an evidence‐based medicine review. Movement Disorders, 34(2), 180-198.
View at Publisher | View at Google Scholar - Song, J., & Kim, Y. K. (2021). Animal models for the study of depressive disorder. CNS neuroscience & therapeutics, 27(6), 633-642.
View at Publisher | View at Google Scholar - Wassmann, C. (2010). Reflections on the “body loop”: Carl Georg Lange's theory of emotion. Cognition and emotion, 24(6), 974-990.
View at Publisher | View at Google Scholar - Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 138(3479), 32-32.
View at Publisher | View at Google Scholar - Lazarus, R. S., Speisman, J. C., & Mordkoff, A. M. (1963). The relationship between autonomic indicators of psychological stress: Heart rate and skin conductance. Psychosomatic medicine, 25(1), 19-30.
View at Publisher | View at Google Scholar - Beck, A. T. (1963). Thinking and depression: I. Idiosyncratic content and cognitive distortions. Archives of general psychiatry, 9(4), 324-333.
View at Publisher | View at Google Scholar - Fiest, K. M., Walker, J. R., Bernstein, C. N., Graff, L. A., Zarychanski, R., Abou-Setta, A. M., ... & Marrie, R. A. (2016). Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Multiple sclerosis and related disorders, 5, 12-26.
View at Publisher | View at Google Scholar - World Health Organization. (2004). International Statistical Classification of Diseases and related health problems: Alphabetical index (Vol. 3). World Health Organization.
View at Publisher | View at Google Scholar - Guha, M. (2014). Diagnostic and statistical manual of mental disorders: DSM-5. Reference Reviews, 28(3), 36-37.
View at Publisher | View at Google Scholar - Pizzi, C., Rutjes, A. W. S., Costa, G. M., Fontana, F., Mezzetti, A., & Manzoli, L. (2011). Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. The American journal of cardiology, 107(7), 972-979.
View at Publisher | View at Google Scholar - Karrouri, R., Hammani, Z., Benjelloun, R., & Otheman, Y. (2021). Major depressive disorder: Validated treatments and future challenges. World journal of clinical cases, 9(31), 9350.
View at Publisher | View at Google Scholar - Wu, Y. H., Chen, Y. H., Chang, M. H., & Lin, C. H. (2018). Depression in Parkinson's disease: A case-control study. PLoS One, 13(2), e0192050.
View at Publisher | View at Google Scholar - Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., ... & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695-699.
View at Publisher | View at Google Scholar - Dubois, B., Slachevsky, A., Litvan, I., & Pillon, B. F. A. B. (2000). The FAB: a frontal assessment battery at bedside. Neurology, 55(11), 1621-1626.
View at Publisher | View at Google Scholar - Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of general psychiatry, 4(6), 561-571.
View at Publisher | View at Google Scholar - Schwarzer, R., & Jerusalem, M. (1995). Generalized self-efficacy scale. J. Weinman, S. Wright, & M. Johnston, Measures in health psychology: A user’s portfolio. Causal and control beliefs, 35, 37.
View at Publisher | View at Google Scholar - Thompson, E. (2015). Hamilton rating scale for anxiety (HAM-A). Occupational Medicine, 65(7), 601-601.
View at Publisher | View at Google Scholar - Derogatis, L. R., & Savitz, K. L. (1999). The SCL-90-R, Brief Symptom Inventory, and Matching Clinical Rating Scales.
View at Publisher | View at Google Scholar - Berg, K., Wood-Dauphinee, S. L., Williams, J. I., & Gayton, D. (1989). Berg Balance Scale (BBS) [Database record]. APA PsycTests.
View at Publisher | View at Google Scholar - Voon, V., Mehta, A. R., & Hallett, M. (2011). Impulse control disorders in Parkinson’s disease: recent advances. Current opinion in neurology, 24(4), 324.
View at Publisher | View at Google Scholar - Vela, L., Castrillo, J. M., Ruiz, P. G., Gasca-Salas, C., Macías, Y. M., Fernández, E. P., ... & Marasescu, R. (2016). The high prevalence of impulse control behaviors in patients with early-onset Parkinson's disease: a cross-sectional multicenter study. Journal of the neurological sciences, 368, 150-154.
View at Publisher | View at Google Scholar - Weintraub, D., Papay, K., Siderowf, A., & Parkinson's Progression Markers Initiative. (2013). Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology, 80(2), 176-180.
View at Publisher | View at Google Scholar - Moore, T. J., Glenmullen, J., & Mattison, D. R. (2014). Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA internal medicine, 174(12), 1930-1933.
View at Publisher | View at Google Scholar - Seeman, P. (2015). Parkinson's disease treatment may cause impulse–control disorder via dopamine D3 receptors. Synapse, 69(4), 183-189.
View at Publisher | View at Google Scholar - Garcia-Ruiz, P. J. (2018). Impulse control disorders and dopamine-related creativity: pathogenesis and mechanism, short review, and hypothesis. Frontiers in neurology, 9, 1041.
View at Publisher | View at Google Scholar - Aiken, C. B. (2007). Pramipexole in psychiatry: a systematic review of the literature. Journal of Clinical Psychiatry, 68(8), 1230-1236.
View at Publisher | View at Google Scholar - Petersen, K., Van Wouwe, N., Stark, A., Lin, Y. C., Kang, H., Trujillo‐Diaz, P., ... & Claassen, D. O. (2018). Ventral striatal network connectivity reflects reward learning and behavior in patients with P arkinson's disease. Human brain mapping, 39(1), 509-521.
View at Publisher | View at Google Scholar - Corvol, J. C., Artaud, F., Cormier-Dequaire, F., Rascol, O., Durif, F., Derkinderen, P., ... & DIGPD Study Group. (2018). Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology, 91(3), e189-e201.
View at Publisher | View at Google Scholar - Mamikonyan, E., Siderowf, A. D., Duda, J. E., Potenza, M. N., Horn, S., Stern, M. B., & Weintraub, D. (2008). Long‐term follow‐up of impulse control disorders in Parkinson's disease. Movement Disorders, 23(1), 75-80.
View at Publisher | View at Google Scholar - Rabinak, C. A., & Nirenberg, M. J. (2010). Dopamine agonist withdrawal syndrome in Parkinson disease. Archives of Neurology, 67(1), 58-63.
View at Publisher | View at Google Scholar - Cunnington, A. L., White, L., & Hood, K. (2012). Identification of possible risk factors for the development of dopamine agonist withdrawal syndrome in Parkinson's disease. Parkinsonism & related disorders, 18(9), 1051-1052.
View at Publisher | View at Google Scholar - Remy, P., Doder, M., Lees, A., Turjanski, N., & Brooks, D. (2005). Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain, 128(6), 1314-1322.
View at Publisher | View at Google Scholar - Pagonabarraga, J., Tinazzi, M., Caccia, C., & Jost, W. H. (2021). The role of glutamatergic neurotransmission in the motor and non-motor symptoms in Parkinson's disease: clinical cases and a review of the literature. Journal of Clinical Neuroscience, 90, 178-183.
View at Publisher | View at Google Scholar - Connolly, B., & Fox, S. H. (2014). Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease. Neurotherapeutics, 11, 78-91.
View at Publisher | View at Google Scholar - Lim, S. Y., Fox, S. H., & Lang, A. E. (2009). Overview of the extranigral aspects of Parkinson disease. Archives of neurology, 66(2), 167-172.
View at Publisher | View at Google Scholar - Cabib, S., & Puglisi-Allegra, S. (1996). Different effects of repeated stressful experiences on mesocortical and mesolimbic dopamine metabolism. Neuroscience, 73(2), 375-380.
View at Publisher | View at Google Scholar - Drevets, W. C. (1998). Functional neuroimaging studies of depression: the anatomy of melancholia. Annual review of medicine, 49(1), 341-361.
View at Publisher | View at Google Scholar - Chen, Z. Q., Du, M. Y., Zhao, Y. J., Huang, X. Q., Li, J., Lui, S., ... & Gong, Q. Y. (2015). Voxel-wise meta-analyses of brain blood flow and local synchrony abnormalities in medication-free patients with major depressive disorder. Journal of Psychiatry and Neuroscience, 40(6), 401-411.
View at Publisher | View at Google Scholar - LeDoux, J. E. (2000). Emotion circuits in the brain. Annual review of neuroscience, 23(1), 155-184.
View at Publisher | View at Google Scholar - McEwen, B. S., Bowles, N. P., Gray, J. D., Hill, M. N., Hunter, R. G., Karatsoreos, I. N., & Nasca, C. (2015). Mechanisms of stress in the brain. Nature neuroscience, 18(10), 1353-1363.
View at Publisher | View at Google Scholar - Fallon, J. H., & Moore, R. Y. (1978). Catecholamine innervation of the basal forebrain IV. Topography of the dopamine projection to the basal forebrain and neostriatum. Journal of Comparative Neurology, 180(3), 545-579.
View at Publisher | View at Google Scholar - Fudge, J. L., & Emiliano, A. B. (2003). The extended amygdala and the dopamine system: another piece of the dopamine puzzle. The Journal of neuropsychiatry and clinical neurosciences, 15(3), 306-316.
View at Publisher | View at Google Scholar - Moore, R. Y. (2003). Organization of midbrain dopamine systems and the pathophysiology of Parkinson's disease. Parkinsonism & related disorders, 9, 65-71.
View at Publisher | View at Google Scholar - Harding, A. J., Stimson, E., Henderson, J. M., & Halliday, G. M. (2002). Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain, 125(11), 2431-2445.
View at Publisher | View at Google Scholar - Prange, S., Klinger, H., Laurencin, C., Danaila, T., & Thobois, S. (2022). Depression in patients with Parkinson’s disease: current understanding of its neurobiology and implications for treatment. Drugs & Aging, 39(6), 417-439.
View at Publisher | View at Google Scholar - Devos, D., Dujardin, K., Poirot, I., Moreau, C., Cottencin, O., Thomas, P., ... & Defebvre, L. (2008). Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: a double‐blind, randomized, placebo‐controlled study. Movement Disorders, 23(6), 850-857.
View at Publisher | View at Google Scholar - Richard, I. H., McDermott, M. P., Kurlan, R., Lyness, J. M., Como, P. G., Pearson, N., ... & Kompoliti, K. (2012). A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology, 78(16), 1229-1236.
View at Publisher | View at Google Scholar - Zhuo, C., Xue, R., Luo, L., Ji, F., Tian, H., Qu, H., ... & Tao, R. (2017). Efficacy of antidepressive medication for depression in Parkinson disease: a network meta-analysis. Medicine, 96(22).
View at Publisher | View at Google Scholar - Wu, P. L., Lee, M., & Huang, T. T. (2017). Effectiveness of physical activity on patients with depression and Parkinson's disease: A systematic review. PloS one, 12(7), e0181515.
View at Publisher | View at Google Scholar - Sacheli, M. A., Neva, J. L., Lakhani, B., Murray, D. K., Vafai, N., Shahinfard, E., ... & Stoessl, A. J. (2019). Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Movement Disorders, 34(12), 1891-1900.
View at Publisher | View at Google Scholar - Sagarwala, R., & Nasrallah, H. A. (2020). The effects of yoga on depression and motor function in patients with Parkinson's disease: A review of controlled studies. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 32(3), 209-215.
View at Publisher | View at Google Scholar - Speelman, A. D., Van De Warrenburg, B. P., Van Nimwegen, M., Petzinger, G. M., Munneke, M., & Bloem, B. R. (2011). How might physical activity benefit patients with Parkinson disease?. Nature Reviews Neurology, 7(9), 528-534.
View at Publisher | View at Google Scholar - Ahmad, M. H., Rizvi, M. A., Ali, M., & Mondal, A. C. (2023). Neurobiology of depression in Parkinson’s disease: Insights into epidemiology, molecular mechanisms and treatment strategies. Ageing research reviews, 101840.
View at Publisher | View at Google Scholar - Weintraub, D., & Claassen, D. O. (2017). Impulse control and related disorders in Parkinson's disease. International review of neurobiology, 133, 679-717.
View at Publisher | View at Google Scholar - Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: onset, progression, and mortality. Neurology, 17(5), 427-427.
View at Publisher | View at Google Scholar