Review Article | DOI: https://doi.org/10.58489/2836-8851/011
Cognitive dysfunction: An important feature of major depressive disorder
- Department of Physiology and Neurobiology, School of Basic Medical Sciences, Zhengzhou University, 100 Science Avenue, Zhengzhou, Henan 450001, China
- Department of Neurology, The First Affiliated Hospital of Zhengzhou University, 40 Daxue Road, Zhengzhou, Henan 450052, China
- The Academy of Medical Sciences, Zhengzhou University, 40 Daxue Road, Zhengzhou, Henan 450052, China
*Corresponding Author: Dong-Xiao Duan
Citation: Xiao-Hui Li, Xuemin Cui, Wang Wang, Lin Yang, Hong Can Zhu, Dong Xiao Duan. (2023). Cognitive dysfunction: An important feature of major depressive disorder. Journal of Neurons and Neurological Disorders. DOI: 10.58489/2836-8851/011
Copyright: © 2023: Dong Xiao Duan, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 11 May 2023 | Accepted: 23 May 2023 | Published: 16 June 2023
Keywords: cognitive dysfunction; major depressive disorder; Physical therapy; Psychotherapy; hippocampus; Exercise therapy
Abstract
Depression is a common mental illness with high prevalence, disability, and suicide rates. Most patients experience recurrent attacks, which converts depression into a chronic disease. Major depressive disorder (MDD) is a type of emotional dysfunction caused by genetic abnormalities or great changes in the patient’s environment, which can manifest as a host of symptoms such as persistent and spontaneous depression with changes in cognitive function. According to worldwide statistics, 50% of suicides each year occur during episodes of depression, and patients with MDD are 20 times more likely to commit suicide than the general population.
Introduction
Depression is a common mental illness with high prevalence, disability, and suicide rates. Most patients experience recurrent attacks, which converts depression into a chronic disease. Major depressive disorder (MDD) is a type of emotional dysfunction caused by genetic abnormalities or great changes in the patient’s environment, which can manifest as a host of symptoms such as persistent and spontaneous depression with changes in cognitive function. According to worldwide statistics, 50% of suicides each year occur during episodes of depression, and patients with MDD are 20 times more likely to commit suicide than the general population. During MDD, patients display obvious changes, such as inattention and memory loss, which are mainly manifested as deficiencies in executive function, memory, learning, language processing, and attention. Persistent symptoms seriously affect patients’ social communication, family life, study, and work, as well as the prognosis of patients and the recovery of social function. However, the mechanisms underlying cognitive dysfunction remain unclear. Therefore, the etiological understanding and treatment of MDD are urgent problems to be solved, in which understanding cognitive dysfunction is an important goal for the diagnosis and treatment of patients with MDD. This review mainly expounds on the etiological and pathological changes and the treatment of cognitive function in major depressive disorder.
Etiology of major depressive disorder
MDD is characterized by one or more severe depressive episodes with significant changes in emotional, cognitive, and autonomic functions; significant weight loss or gain; fatigue; feeling worthless or guilty; inability to concentrate; and/or recurrent suicidal thoughts lasting for at least two [Error! Reference source not found.,1]. The incidence of MDD in women is twice as high as that in men, with one in six adults suffering from the disease. The pathogenesis of MDD has not been fully elucidated because it involves the interaction between complex genetic and environmental factors; hence, its etiological mechanism has been the focus of much research.
The main causes of MDD are as follows (Figure.1)
1) Personality traits: The negative components of personality, such as inferiority, remorse, and pessimism, can easily manifest in MDD; however, the risk of MDD can be reduced through daily psychological adjustment. Once such a patient is diagnosed with MDD, the treatment time is prolonged, and most patients need pharmacological treatment combined with psychotherapy to completely eliminate the symptoms [1,4].
2) Genetic factors: MDD has obvious hereditary features. If someone in the family suffers from MDD, especially immediate family members, the likelihood of a person developing MDD will increase. Children whose parents have MDD have a 35–37% chance of developing depression. In addition, a genetic crossover between MDD and other mental disorders (such as schizophrenia and bipolar disorder) has been identified [1,5,6].
3) Disease factors: Studies have shown that MDD is closely associated with diabetes, heart disease, cancer, Alzheimer’s disease, and stroke. If underlying conditions are present, the incidence of MDD increases. For example, about one in five patients with cardiovascular disease have MDD, and MDD is a risk factor for the incidence, severity, and poor prognosis of cardiovascular disease [7,8,9,10].
4) Environmental factors and adverse stimuli: When environmental stress is too high or patients experience adverse stimulation, the incidence of MDD increases [11,12]. MDD-related stress events usually occur one year before the onset of the disease, including unemployment, economic stress, chronic or life-threatening health problems, violence, separation, and bereavement, which often occur in adulthood [13,14]. However, Dannehl et al. found that children who experienced physical and sexual abuse, psychological injury, domestic violence, or premature separation from their parents were more likely to develop MDD later in life. The number and severity of adverse life events were positively correlated with the risk, severity, and chronic duration of MDD [14,15]. In most cases, the etiology of MDD is not singular, and different causes may exist simultaneously, augmenting each other’s effect on MDD occurrence and development. MDD will, in turn, affect its etiology. For example, patients with MDD are generally less able to withstand stress and are more likely to suffer from certain diseases than healthy people, and MDD also impedes the treatment of other diseases, such as depression associated with Alzheimer’s disease [16,17].
Cognitive Dysfunction and pathological changes in MDD
Studies have shown that patients with depression have defects in the following cognitive areas: executive function and attention transfer, memory, visual-spatial processing, and psychomotor function [18,19,20]. At present, cognitive dysfunction in depression is a primary focus of MDD research and is a potential predictor of occupational and social adaptation disorders in adult MDD patients. After the psychological symptoms of MDD are relieved, cognitive dysfunction persists and needs to be treated separately from emotional symptoms [21,22,23]. It is worth noting that patients’ cognitive deficits may affect their professional and social communication abilities and lead to suicidal ideation. Additionally, the persistence of cognitive dysfunction may interact with existing depression and increase the risk of recurrence [24,25].
The brain is a complex neural network with interconnections between brain regions. Studies have found that some neural network functions change during depression, including those of the prefrontal cortex and cingulate gyrus, subcortical areas of the striatum and thalamus, and temporal cortex, including the amygdala and hippocampus [26]. Lesions in the prefrontal cortex will cause a decrease in executive function, and a decrease in hippocampal volume will lead to memory impairment, which may be the progressive result of MD [25,26]. Therefore, restoring the function of these structures may become the basis for the treatment of depression. However, existing treatment methods are not effective for all cases, and it is necessary to further understand the relationship between cognitive dysfunction and neural markers of MDD. Therefore, research and treatment of cognitive dysfunction in MDD are very important.
Initially, depression was thought to be only a mental disorder; however, with an increase in research on depression, a number of studies have found that patients with MDD have organic lesions. The cross-sectional results of brain structural imaging showed that the main brain regions related to MDD pathogenesis were the frontal cortex, thalamus, striatum, hippocampus, and parietal cortex [27]. Magnetic resonance imaging studies found that the volume of many brain regions decreased during MDD; in particular, hippocampal volume reduction was consistently observed, but it is not clear whether hippocampal volume reduction is an early manifestation or a late phenomenon in the course of the disease [28]. The hippocampus is involved in the memory, recall, and reward systems. There is evidence that when patients with MDD are stressed, the level of glucocorticoids in the hippocampus increases through the hypothalamus-pituitary-adrenal axis; glucocorticoids then bind to the glucocorticoid receptor in the hippocampus, resulting in hippocampal neuronal atrophy [29]. A study of antidepressant therapy and electroconvulsive therapy in patients with MDD showed that the decrease in hippocampal gray matter volume and hippocampal functional activity in patients with depression led to a decrease in negative emotion and cognitive ability, suggesting that the increase in hippocampal gray matter volume during therapy was related to the improvement of clinical symptoms[30,31,32,33].
The striatum is an important component of the basal ganglia. Neuroimaging studies have reported significant changes in the striatum of patients with MDD and decreased gray matter intensity in the ventral striatum of patients with MDD who committed suicide Major depressive disorder (MDD) causes great decrements in health and quality of life with increments in healthcare costs, but the causes and pathogenesis of depression remain largely unknown, which greatly prevent its early detection and effective treatment. With the advancement of neuroimaging approaches, numerous functional and structural alterations in the brain have been detected in MDD and more recently attempts have been made to apply these findings to clinical practice. In this review, we provide an updated summary of the progress in the translational application of psycho-radiological findings in MDD with a specified focus on potential clinical usage. The foreseeable clinical applications for different MRI modalities were introduced according to their role in disorder classification, subtyping, and prediction. While evidence of cerebral structural and functional changes associated with MDD classification and subtyping was heterogeneous and/or sparse, the ACC and hippocampus have been consistently suggested to be important biomarkers in predicting treatment selection and treatment response. These findings underlined the potential utility of brain biomarkers for clinical practice. The interruption of striatal output is speculated to lead to impulsive suicidal behavior. Functional magnetic resonance imaging showed a decrease in striatal activity in the reward system, and the decrease in reward network connections was related to the severity of depression. These findings suggest that abnormal striatal activity plays an important role in the disease progression [34,35]. The literature shows that compared with healthy adults, the volume of the bilateral putamen, caudate nucleus, right amygdala, and left thalamus in MDD patients is significantly reduced [36,37,38,39]. The putamen plays a key role in emotion, cognitive processes, motivation, and motor regulation, whereas the dysfunction of the caudate nucleus may lead to the interruption of dopaminergic signals and reduce the afferent impulses of ventral tegmental dopaminergic neurons [39,40,41].
The pathological changes in different brain regions of patients with MDD may correspond to the symptoms; however, these alterations are difficult to use as the basis for treatment. At present, research on the role of each brain region in MDD is incomplete, and it is difficult to treat one brain region alone.
Treatment of MDD
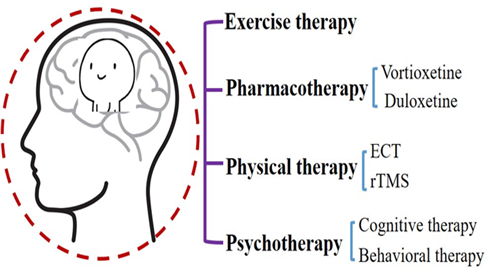
Many hypotheses have been proposed for the treatment of MDD, including the stress-induced inflammatory factors hypothesis, hypothalamus-pituitary-adrenocortical axis hypertrophy hypothesis, and metabolic abnormality-induced changes in neurotransmitters and neurotrophic factors hypothesis, among others. Changes that can lead to an increase in inflammatory factors in the neural system are an imbalance between the synthesis and release of neurotransmitters, a decrease in the secretion of brain-derived nerve growth factor (BDNF), a decrease in the protective effect on nerve cells, and the loss of cortical and hippocampal neurons. These circumstances lead to a decline in hippocampal function and cognitive dysfunction. However, the detailed mechanism has not been fully clarified, and the corresponding treatment has not been developed. Currently, the main treatment methods for cognitive function are drug therapy, physiotherapy, psychotherapy, and exercise therapy (Figure.2).
Pharmacotherapy for MDD
Many MDD patients have improved moods after antidepressant treatment, but the improvement in cognitive function is not necessarily consistent. It has been proven that persistent cognitive dysfunction after the remission of depressive symptoms is the reason why patients cannot fully recover, and vice versa; dysfunction can also lead to the persistence of cognitive symptoms in MDD. To date, two antidepressants, vortioxetine, and duloxetine, have been shown to cause direct, independent, and clinically related changes in cognitive dysfunction in patients with MDD [42,43]. Vortioxetine seems to have the greatest effect on psychomotor speed, executive control, and cognitive control, whereas duloxetine has the greatest effect on delayed recall [23,44,45].
Among the antidepressants, vortioxetine is the most widely studied [44.45,46]. It is an effective and well-tolerated antidepressant that inhibits serotonin (5-HT or 5-hydroxytryptamine) transporters and regulates a variety of 5-HT receptors [48,49]. Antidepressants administered through multiple pathways affect multiple neurotransmitter systems, including serotonin, norepinephrine, dopamine, gamma-aminobutyric acid, glutamic acid, histamine, and the cholinergic system [50,51,52]. Previous clinical studies have shown that vortioxetine is an antagonist of 5-HT1D, 5-HT3, and 5-HT7 receptors and an inhibitor of some, but not all, 5-HT1A and 5-HT1B receptor agonists and 5-HT transporters [1]. The inhibition of the serotonin transporter 5-HTT and the activation of 5-HT1A receptors induces a rapid and powerful antidepressant response, and the action of 5-HT3 receptors can increase the release of norepinephrine and acetylcholine in the forebrain and improve cognitive dysfunction in MDD [53]. Vortioxetine can increase extracellular 5-HT levels and induce adult hippocampal neurogenesis, which is a candidate mechanism for mediating antidepressant responses in rodents [54]. Acute administration of vortioxetine can reverse the memory impairment caused by a 5-HT loss in rats in a dose-dependent manner, and long-term treatment can reverse the memory impairment caused by a 5-HT loss in rats. Recent studies have shown that acute and subchronic intermittent administration of vortioxetine can improve the reversal of learning disorder induced by 4-chloro-DL-phenylalanine methyl ester hydrochloride, used to reduce serotonin in rats [545556]. In addition, the long-term use of vortioxetine can revert the reversal of learning impairment caused by chronic intermittent cold stress in normal animals. Therefore, based on the available data, vortioxetine is a useful treatment option for patients with MDD and significant cognitive dysfunction.
Physical Therapy for MDD
Physiotherapy for MDD mainly includes electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS). ECT is an effective and safe treatment with proven success in the treatment of MDD [57]. However, the exact underlying mechanism remains unclear. Its antidepressant effect on changing the level of neurotransmitters, improving neuroplasticity, and increasing functional connectivity is related to BDNF levels [58]. ECT is usually recommended as a second-line treatment for depression because of its potential side effects such as transient memory impairment, disturbance of consciousness, headache, and nausea [59,60].
rTMS was approved by the United States Food and Drug Administration in 2008 for the treatment of major depression. It has been increasingly used clinically worldwide [61]. Experiments have shown that cranial magnetic stimulation can change the excitability of the stimulated areas of the brain, which lasts longer than the stimulation itself. From a therapeutic perspective, this feature of rTMS is particularly beneficial. Depending on the frequency of stimulation, the activity of the cortex becomes excitatory or inhibitory. When repetitive pulses stimulate the primary motor cortex, low-frequency stimulation (1 Hz) usually causes neuronal inhibition, whereas high-frequency stimulation (10–20 Hz) induces neuronal excitability [62,63]. The main target of rTMS in the treatment of mental disorders is the dorsolateral prefrontal cortex (DLPFC). Studies have shown that stimulation of the DLPFC by rTMS is a safe and effective alternative treatment for intractable depression [64,65]. The basic principle of MDD targeting the DLPFC is based on functional neuroimaging studies that show reduced activity in the left prefrontal lobe. The DLPFC is also an important node in the dorsal executive system, which is involved in cognitive control in emotion regulation. Cognitive control functions include selective attention, inhibitory control, and cognitive re-evaluation. The role of the DLPFC in regulating these functions has been supported by previous research [65,66]. Although rTMS has made many achievements in the treatment of cognitive function in MDD, the efficacy in many patients is different because of the heterogeneity of MDD. Many aspects of current research are unraveling the mechanism of rTMS itself; however, rTMS is still very promising for the treatment of MDD.
Psychotherapy for MDD
The recurrence rate of MDD is very high; with each repeated attack, the depression situation worsens, and the risk of the next recurrence increases [67,68,69]. One study found that the risk of recurrence after one episode of major depression was 50% and 80
Exercise Therapy for MDD
Since no single treatment is effective for patients, there has been great interest in the development and evaluation of alternative therapies for MDD, and physical exercise is one of the treatments that has received considerable attention [79,80]. Studies have shown that the beneficial effects of exercise on depression depend on the regulation of neurotransmitters, neurogenesis, neurotrophic factors, and cerebral blood flow [81]. It has been reported that exercise can induce an increase in the level of BDNF and helps improve the ability of mice to resist anxiety and depression [82]. D-β-hydroxybutyrate can pass through the blood-brain barrier, and the possible mechanism underlying the effect of exercise is that the accumulation of endogenous D-β-hydroxybutyrate in the hippocampus inhibits class I histone deacetylase, specifically increases the expression of BDNF, and affects synaptic transmission [82]. In a mouse model of depression caused by sleep deprivation, exercise increased the level of BDNF, thus playing a neuro-protective and neurotrophic role. Experiments have shown that BDNF can be increased by exercise, which regulates synaptic plasticity by acting on axon and dendritic remodeling, synaptogenesis, and synaptic function [82,83]. BDNF activity is speculated to be closely related to learning and memory function. In addition, studies have shown that exercise preconditioning can prevent depressive behavior and changes in neurotransmitters such as increased levels of noradrenaline, serotonin, and their metabolites in the brains of a sleep-deprived mouse depression model [83,84,85]. In addition, in an animal model of depression induced by maternal separation, the levels of 5-hydroxytryptamine and tryptophan hydroxylase in the dorsal raphe decreased, whereas exercise alleviated depression-like behavior by increasing the expression of these factors [86,87].
Although the etiology of MDD is unclear, many factors have been found to affect the development of the disease, such as changes in neurotransmitters, decreased synaptic plasticity, and reduced hippocampal size. The hippocampus plays an indispensable role in learning and memory, has a high degree of neural plasticity, and is one of only two areas of the brain that show adult neurogenesis (the other being the olfactory bulb) [88,89,90]. Exercise has been shown to increase the volume and blood flow of the hippocampus, stimulate neurogenesis, regulate synaptic plasticity, and increase the expression of growth factors, such as BDNF, which optimize brain function [81]. There is no direct comparison between pharmacological treatments and exercise therapy for cognitive dysfunction therefore, there is no way to quantify the effect of exercise to improve cognitive dysfunction [91,92].
Physical exercise can be used as an auxiliary and individualized treatment for MDD [93,94] or as a strategy to prevent MDD [95]. Physical exercise has a positive effect on synaptic plasticity and neurogenesis via various biological mechanisms [96,97]. Patients with MDD participate less in physical activity, show lower cardiopulmonary adaptability, and have an increased risk of metabolic diseases and premature death [98]. From another point of view, it should be noted that a large number of acute exercises are stressful events, and the intensity of acute exercise determines the magnitude of stress [99], therefore, patients with MDD should be aware of exercise intensity and try to exercise aerobically [99,101].
Conclusion
Although tremendous progress has been made in predicting, diagnosing, and ameliorating the symptoms of MDD, it remains a serious disease that imposes a heavy burden on both individuals and society [102]. In the treatment of MDD, the widely accepted outcome is symptomatic remission; that is, patients return to pre-ictal functional levels with no or only mild depressive symptoms. Combination therapy is currently advocated for MDD treatment [103]. Studies have found that, after antidepressants are administered in the acute phase of MDD, psychotherapy seems to be an effective strategy, and the effect is ideal in preventing relapse [68104]. There are still many unresolved problems associated with the combined treatment of MDD. For example, there are different treatment methods recommended for use in the acute exacerbation and remission periods of MDD; there is controversy as to which should be used as the main treatment, which should be adjuvant treatment, and when adjuvant treatment should be carried out [105]. However, these problems are significant. Therefore, an in-depth understanding of MDD is very important, and there is a long way to go before MDD can be successfully treated.
Acknowledgement
This study was supported by the Science and Technology Department of Henan Province (Project No. 192102310084 and 222102310143) and the Youth Fund of Basic Medical School, Zhengzhou University (Project No: JCYXY2017-YQ-07).
Conflict of interests
The authors declare that they have no conflicts of interest.
References
- Read, J. R., Sharpe, L., Modini, M., & Dear, B. F. (2017). Multimorbidity and depression: a systematic review and meta-analysis. Journal of affective disorders, 221, 36-46.
View at Publisher | View at Google Scholar - Ménard, C., Hodes, G. E., & Russo, S. J. (2016). Pathogenesis of depression: insights from human and rodent studies. Neuroscience, 321, 138-162.
View at Publisher | View at Google Scholar - Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., ... & Schatzberg, A. F. (2016). Major depressive disorder. Nature reviews Disease primers, 2(1), 1-20.
View at Publisher | View at Google Scholar - Balestri, M., Porcelli, S., Souery, D., Kasper, S., Dikeos, D., Ferentinos, P., Papadimitriou, G. N., Rujescu, D., Martinotti, G., Di Nicola, M., Janiri, L., Caletti, E., Mandolini, G. M., Pigoni, A., Paoli, R. A., Lazzaretti, M., Brambilla, P., Sala, M., Abbiati, V., Bellani, M., Serretti, A. 2019. Temperament and character influence on depression treatment outcome. Journal of affective disorders, 252, 464–474.
View at Publisher | View at Google Scholar - Snijders, G., Mesman, E., de Wit, H., Wijkhuijs, A., Nolen, W. A., Drexhage, H. A., Hillegers, M. 2017. Immune dysregulation in offspring of a bipolar parent. Altered serum levels of immune growth factors at adolescent age. Brain, behavior, and immunity, 64, 116–123.
View at Publisher | View at Google Scholar - Goodday, S. M., Horrocks, J., Keown-Stoneman, C., Grof, P., Duffy, A. 2016. Repeated salivary daytime cortisol and onset of mood episodes in offspring of bipolar parents. International journal of bipolar disorders, 4(1), 12.
View at Publisher | View at Google Scholar - Lichtman, J. H., Froelicher, E. S., Blumenthal, J. A., Carney, R. M., Doering, L. V., Frasure-Smith, N., Freedland, K. E., Jaffe, A. S., Leifheit-Limson, E. C., Sheps, D. S., Vaccarino, V., Wulsin, L., 2014. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation, 129(12), 1350–1369.
View at Publisher | View at Google Scholar - Elderon, L., Whooley, M. A. 2013. Depression and cardiovascular disease. Progress in cardiovascular diseases, 55(6), 511–523.
View at Publisher | View at Google Scholar - Gallo, J. J., Bogner, H. R., Morales, K. H., Post, E. P., Ten Have, T., Bruce, M. L. 2005. Depression, cardiovascular disease, diabetes, and two-year mortality among older, primary-care patients. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry, 13(9), 748–755.
View at Publisher | View at Google Scholar - Evans, D. L., Charney, D. S., Lewis, L., Golden, R. N., Gorman, J. M., Krishnan, K. R., Nemeroff, C. B., Bremner, J. D., Carney, R. M., Coyne, J. C., Delong, M. R., Frasure-Smith, N., Glassman, A. H., Gold, P. W., Grant, I., Gwyther, L., Ironson, G., Johnson, R. L., Kanner, A. M., Katon, W. J., Valvo, W. J. 2005. Mood disorders in the medically ill: scientific review and recommendations. Biological psychiatry, 58(3), 175–189.
View at Publisher | View at Google Scholar - Tannous, J., Godlewska, B. R., Tirumalaraju, V., Soares, J. C., Cowen, P. J., Selvaraj, S. 2020. Stress, inflammation and hippocampal subfields in depression: A 7 Tesla MRI Study. Translational psychiatry, 10(1), 78.
View at Publisher | View at Google Scholar - Wang, Y., Sareen, J., Afifi, T. O., Bolton, S. L., Johnson, E. A., Bolton, J. M. 2015. A population-based longitudinal study of recent stressful life events as risk factors for suicidal behavior in major depressive disorder. Archives of suicide research: official journal of the International Academy for Suicide Research, 19(2), 202–217.
View at Publisher | View at Google Scholar - Xie, P., Wu, K., Zheng, Y., Guo, Y., Yang, Y., He, J., Ding, Y., Peng, H. 2018. Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. Journal of affective disorders, 228, 41–48.
View at Publisher | View at Google Scholar - Wainsztein, A. E., Castro, M. N., Goldberg, X., Camacho-Téllez, V., Vulcano, M., Abulafia, C., Ladrón-de-Guevara, S., Cardoner, N., Nemeroff, C. B., Menchón, J. M., Soriano-Mas, C., Villarreal, M. F., Guinjoan, S. M. 2021. Childhood adversity modulation of central autonomic network components during cognitive regulation of emotion in major depressive disorder and borderline personality disorder. Psychiatry research. Neuroimaging, 318, 111394.
View at Publisher | View at Google Scholar - Dannehl, K., Rief, W., Euteneuer, F. 2017. Childhood adversity and cognitive functioning in patients with major depression. Child abuse & neglect, 70, 247–254.
View at Publisher | View at Google Scholar - Kuo, C. Y., Stachiv, I., Nikolai, T. 2020. Association of Late Life Depression, (Non-) Modifiable Risk and Protective Factors with Dementia and Alzheimer's Disease: Literature Review on Current Evidences, Preventive Interventions and Possible Future Trends in Prevention and Treatment of Dementia. International journal of environmental research and public health, 17(20), 7475.
View at Publisher | View at Google Scholar - Galts, C., Bettio, L., Jewett, D. C., Yang, C. C., Brocardo, P. S., Rodrigues, A., Thacker, J. S., Gil-Mohapel, J. 2019. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neuroscience and biobehavioral reviews, 102, 56–84.
View at Publisher | View at Google Scholar - Czerwińska, A., Pawłowski, T. 2020. Cognitive dysfunctions in depression - significance, description and treatment prospects. Zaburzenia funkcji poznawczych w depresji – znaczenie, charakterystyka oraz możliwości leczenia. Psychiatria polska, 54(3), 453–466.
View at Publisher | View at Google Scholar - Rubin, Rita. 2018. Exploring the Relationship Between Depression and Dementia. JAMA, 320, 961-962.
View at Publisher | View at Google Scholar - Marazziti, D., Consoli, G., Picchetti, M., Carlini, M., Faravelli, L. 2010. Cognitive impairment in major depression. European journal of pharmacology, 626(1), 83–86.
View at Publisher | View at Google Scholar - Knight, M. J., Baune, B. T. 2018. Cognitive dysfunction in major depressive disorder. Current opinion in psychiatry, 31(1), 26–31.
View at Publisher | View at Google Scholar - Hori, H., Yamato, K. 2019. Assessment of current clinical practices for major depression in Japan using a web-based questionnaire. Neuropsychiatric disease and treatment, 15, 2821–2832.
View at Publisher | View at Google Scholar - Zuckerman, H., Pan, Z., Park, C., Brietzke, E., Musial, N., Shariq, A. S., Iacobucci, M., Yim, S. J., Lui, L., Rong, C., McIntyre, R. S. 2018. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Frontiers in psychiatry, 9, 655.
View at Publisher | View at Google Scholar - Lorenzo-Luaces, L., German, R. E., DeRubeis, R. J. 2015. It's complicated: The relation between cognitive change procedures, cognitive change, and symptom change in cognitive therapy for depression. Clinical psychology review, 41, 3–15.
View at Publisher | View at Google Scholar - Disner, S. G., Beevers, C. G., Haigh, E. A., Beck, A. T. 2011. Neural mechanisms of the cognitive model of depression. Nature reviews. Neuroscience, 12(8), 467–477.
View at Publisher | View at Google Scholar - Clark, L., Chamberlain, S. R., Sahakian, B. J. 2009. Neurocognitive mechanisms in depression: implications for treatment. Annual review of neuroscience, 32, 57–74.
View at Publisher | View at Google Scholar - Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., Gong, Q. Y. 2018. Brain structure alterations in depression: Psychoradiological evidence. CNS neuroscience & therapeutics, 24(11), 994–1003.
View at Publisher | View at Google Scholar - Malykhin, N. V., Coupland, N. J. 2015. Hippocampal neuroplasticity in major depressive disorder. Neuroscience, 309, 200–213.
View at Publisher | View at Google Scholar - Bani-Fatemi, A., Tasmim, S., Graff-Guerrero, A., Gerretsen, P., Strauss, J., Kolla, N., Spalletta, G., De Luca, V. 2018. Structural and functional alterations of the suicidal brain: An updated review of neuroimaging studies. Psychiatry research. Neuroimaging, 278, 77–91.
View at Publisher | View at Google Scholar - Perini, G. I., Toffanin, T., Pigato, G., Ferri, G., Follador, H., Zonta, F., Pastorelli, C., Piazzon, G., Denaro, L., Rolma, G., Ermani, M., DʼAvella, D. 2017. Hippocampal Gray Volumes Increase in Treatment-Resistant Depression Responding to Vagus Nerve Stimulation. The journal of ECT, 33(3), 160–166.
View at Publisher | View at Google Scholar - Enneking, V., Dzvonyar, F., Dannlowski, U., Redlich, R. 2019. Neuronale Effekte und Biomarker antidepressiver Therapieverfahren: Aktueller Überblick aus der Perspektive der neuronalen Bildgebung [Neuronal effects and biomarkers of antidepressant treatments: Current review from the perspective of neuroimaging]. Der Nervenarzt, 90(3), 319–329.
View at Publisher | View at Google Scholar - Gyger, L., Regen, F., Ramponi, C., Marquis, R., Mall, J. F., Swierkosz-Lenart, K., von Gunten, A., Toni, N., Kherif, F., Heuser, I., Draganski, B. 2021. Gradient of electro-convulsive therapy's antidepressant effects along the longitudinal hippocampal axis. Translational psychiatry, 11(1), 191.
View at Publisher | View at Google Scholar - Leaver, A. M., Vasavada, M., Kubicki, A., Wade, B., Loureiro, J., Hellemann, G., Joshi, S. H., Woods, R. P., Espinoza, R., Narr, K. L. 2021. Hippocampal subregions and networks linked with antidepressant response to electroconvulsive therapy. Molecular psychiatry, 26(8), 4288–4299.
View at Publisher | View at Google Scholar - Arrondo, G., Segarra, N., Metastasio, A., Ziauddeen, H., Spencer, J., Reinders, N. R., Dudas, R. B., Robbins, T. W., Fletcher, P. C., Murray, G. K. 2015. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Frontiers in psychology, 6, 1280.
View at Publisher | View at Google Scholar - Gaillard, C., Guillod, M., Ernst, M., Federspiel, A., Schoebi, D., Recabarren, R. E., Ouyang, X., Mueller-Pfeiffer, C., Horsch, A., Homan, P., Wiest, R., Hasler, G., Martin-Soelch, C. 2020. Striatal reactivity to reward under threat-of-shock and working memory load in adults at increased familial risk for major depression: A preliminary study. NeuroImage. Clinical, 26, 102193.
View at Publisher | View at Google Scholar - Zheng, R., Zhang, Y., Yang, Z., Han, S., Cheng, J. 2021. Reduced Brain Gray Matter Volume in Patients With First-Episode Major Depressive Disorder: A Quantitative Meta-Analysis. Frontiers in psychiatry, 12, 671348.
View at Publisher | View at Google Scholar - Ng, T. H., Alloy, L. B., Smith, D. V. 2019. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Translational psychiatry, 9(1), 293.
View at Publisher | View at Google Scholar - Rupprechter, S., Romaniuk, L., Series, P., Hirose, Y., Hawkins, E., Sandu, A. L., Waiter, G. D., McNeil, C. J., Shen, X., Harris, M. A., Campbell, A., Porteous, D., Macfarlane, J. A., Lawrie, S. M., Murray, A. D., Delgado, M. R., McIntosh, A. M., Whalley, H. C., Steele, J. D. 2020. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain: a journal of neurology, 143(6), 1946–1956.
View at Publisher | View at Google Scholar - Johnston, B. A., Steele, J. D., Tolomeo, S., Christmas, D., Matthews, K. 2015. Structural MRI-Based Predictions in Patients with Treatment-Refractory Depression (TRD). PloS one, 10(7), e0132958.
View at Publisher | View at Google Scholar - Sachs-Ericsson, N. J., Hajcak, G., Sheffler, J. L., Stanley, I. H., Selby, E. A., Potter, G. G., Steffens, D. C. 2018. Putamen Volume Differences Among Older Adults: Depression Status, Melancholia, and Age. Journal of geriatric psychiatry and neurology, 31(1), 39–49.
View at Publisher | View at Google Scholar - Nimarko, A. F., Fischer, A. S., Hagan, K. E., Gorelik, A. J., Lu, Y., Young, C. J., Singh, M. K. 2021. Neural Correlates of Positive Emotion Processing That Distinguish Healthy Youths at Familial Risk for Bipolar Versus Major Depressive Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 60(7), 887–901.
View at Publisher | View at Google Scholar - Mahableshwarkar, A. R., Zajecka, J., Jacobson, W., Chen, Y., Keefe, R. S. 2016. A Randomized, Placebo-Controlled, Active-Reference, Double-Blind, Flexible-Dose Study of the Efficacy of Vortioxetine on Cognitive Function in Major Depressive Disorder. Neuropsychopharmacology, 41(12), 2961.
View at Publisher | View at Google Scholar - Waller, J. A., Chen, F., Sánchez, C. 2016. Vortioxetine promotes maturation of dendritic spines in vitro: A comparative study in hippocampal cultures. Neuropharmacology, 103, 143–154.
View at Publisher | View at Google Scholar - Pan, Z., Park, C., Brietzke, E., Zuckerman, H., Rong, C., Mansur, R. B., Fus, D., Subramaniapillai, M., Lee, Y., McIntyre, R. S. 2019. Cognitive impairment in major depressive disorder. CNS spectrums, 24(1), 22–29.
View at Publisher | View at Google Scholar - Bennabi, D., Haffen, E., Van Waes, V. 2019. Vortioxetine for Cognitive Enhancement in Major Depression: From Animal Models to Clinical Research. Frontiers in psychiatry, 10, 771.
View at Publisher | View at Google Scholar - Christensen, M. C., Loft, H., , R. S. 2018. Vortioxetine improves symptomatic and functional outcomes in major depressive disorder: A novel dual outcome measure in depressive disorders. Journal of affective disorders, 227, 787–794.
View at Publisher | View at Google Scholar - McIntyre R. S. 2017. The role of new antidepressants in clinical practice in Canada: a brief review of vortioxetine, levomilnacipran ER, and vilazodone. Neuropsychiatric disease and treatment, 13, 2913–2919.
View at Publisher | View at Google Scholar - Okada, M., Matsumoto, R., Yamamoto, Y., Fukuyama, K. 2021. Effects of Subchronic Administrations of Vortioxetine, Lurasidone, and Escitalopram on Thalamocortical Glutamatergic Transmission Associated with Serotonin 5-HT7 Receptor. International journal of molecular sciences, 22(3), 1351.
View at Publisher | View at Google Scholar - Okada, M., Okubo, R., Fukuyama, K. 2019. Vortioxetine Subchronically Activates Serotonergic Transmission via Desensitization of Serotonin 5-HT1A Receptor with 5-HT3 Receptor Inhibition in Rats. International journal of molecular sciences, 20(24), 6235.
View at Publisher | View at Google Scholar - Cano, G. H., Dean, J., Abreu, S. P., Rodríguez, A. H., Abbasi, C., Hinson, M., & Lucke-Wold, B. 2022. Key Characteristics and Development of Psychoceuticals: A Review. International journal of molecular sciences, 23(24), 15777.
View at Publisher | View at Google Scholar - Nguyen, L., Thomas, K. L., Lucke-Wold, B. P., Cavendish, J. Z., Crowe, M. S., & Matsumoto, R. R. 2016. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacology & therapeutics, 159, 1–22.
View at Publisher | View at Google Scholar - Frampton J. E. 2016. Vortioxetine: A Review in Cognitive Dysfunction in Depression. Drugs, 76(17), 1675–1682.
View at Publisher | View at Google Scholar - David, D. J., Gardier, A. M. 2016. Les bases de pharmacologie fondamentale du système sérotoninergique application à la réponse antidépressive [The pharmacological basis of the serotonin system: Application to antidepressant response]. L'Encephale, 42(3), 255–263.
View at Publisher | View at Google Scholar - Guilloux, J. P., Mendez-David, I., Pehrson, A., Guiard, B. P., Repérant, C., Orvoën, S., Gardier, A. M., Hen, R., Ebert, B., Miller, S., Sanchez, C., David, D. J. 2013. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology, 73, 147–159.
View at Publisher | View at Google Scholar - Mendez-David, I., Hen, R., Gardier, A. M., David, D. J. 2013. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Annales pharmaceutiques francaises, 71(3), 143–149.
View at Publisher | View at Google Scholar - Eisch, A. J., Petrik, D. 2012. Depression and hippocampal neurogenesis: a road to remission?. Science, 338(6103), 72–75.
View at Publisher | View at Google Scholar - van Diermen, L., van den Ameele, S., Kamperman, A. M., Sabbe, B., Vermeulen, T., Schrijvers, D., Birkenhäger, T. K. 2018. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. The British journal of psychiatry: the journal of mental science, 212(2), 71–80.
View at Publisher | View at Google Scholar - Luan, S., Zhou, B., Wu, Q., Wan, H., Li, H. 2020. Brain-derived neurotrophic factor blood levels after electroconvulsive therapy in patients with major depressive disorder: A systematic review and meta-analysis. Asian journal of psychiatry, 51, 101983.
View at Publisher | View at Google Scholar - Kennedy, S. H., Lam, R. W., McIntyre, R. S., Tourjman, S. V., Bhat, V., Blier, P., Hasnain, M., Jollant, F., Levitt, A. J., MacQueen, G. M., McInerney, S. J., McIntosh, D., Milev, R. V., Müller, D. J., Parikh, S. V., Pearson, N. L., Ravindran, A. V., Uher, R., CANMAT Depression Work Group 2016. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 61(9), 540–560.
View at Publisher | View at Google Scholar - Yatham, L. N., Kennedy, S. H., Parikh, S. V., Schaffer, A., Bond, D. J., Frey, B. N., Sharma, V., Goldstein, B. I., Rej, S., Beaulieu, S., Alda, M., MacQueen, G., Milev, R. V., Ravindran, A., O'Donovan, C., McIntosh, D., Lam, R. W., Vazquez, G., Kapczinski, F., McIntyre, R. S., … Berk, M. 2018. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar disorders, 20(2), 97–170.
View at Publisher | View at Google Scholar - McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O'Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., Lisanby, S. H., 2018. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. The Journal of clinical psychiatry, 79(1), 16cs10905.
View at Publisher | View at Google Scholar - Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., Pascual-Leone, A. 2000. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 111(5), 800–805.
View at Publisher | View at Google Scholar - Taylor, J. L., Loo, C. K. 2007. Stimulus waveform influences the efficacy of repetitive transcranial magnetic stimulation. Journal of affective disorders, 97(1-3), 271–276.
View at Publisher | View at Google Scholar - Martin, D. M., McClintock, S. M., Forster, J. J., Lo, T. Y., Loo, C. K. 2017. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depression and anxiety, 34(11), 1029–1039.
View at Publisher | View at Google Scholar - Iimori, T., Nakajima, S., Miyazaki, T., Tarumi, R., Ogyu, K., Wada, M., Tsugawa, S., Masuda, F., Daskalakis, Z. J., Blumberger, D. M., Mimura, M., Noda, Y. 2019. Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer's disease: A systematic review. Progress in neuro-psychopharmacology & biological psychiatry, 88, 31–40.
View at Publisher | View at Google Scholar - Cash, R., Cocchi, L., Lv, J., Fitzgerald, P. B., Zalesky, A. 2021. Functional Magnetic Resonance Imaging-Guided Personalization of Transcranial Magnetic Stimulation Treatment for Depression. JAMA psychiatry, 78(3), 337–339.
View at Publisher | View at Google Scholar - LeMoult, J., Kircanski, K., Prasad, G., Gotlib, I. H. 2017. Negative Self-Referential Processing Predicts the Recurrence of Major Depressive Episodes. Clinical psychological science: a journal of the Association for Psychological Science, 5(1), 174–181.
View at Publisher | View at Google Scholar - Guidi, J., Fava, G. A. 2021. Sequential Combination of Pharmacotherapy and Psychotherapy in Major Depressive Disorder: A Systematic Review and Meta-analysis. JAMA psychiatry, 78(3), 261–269.
View at Publisher | View at Google Scholar - Pitsillou, E., Bresnehan, S. M., Kagarakis, E. A., Wijoyo, S. J., Liang, J., Hung, A., Karagiannis, T. C. 2020. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Molecular biology reports, 47(1), 753–770.
View at Publisher | View at Google Scholar - Altaweel, N., Upthegrove, R., Surtees, A., Durdurak, B., & Marwaha, S. 2023. Personality traits as risk factors for relapse or recurrence in major depression: a systematic review. Frontiers in psychiatry, 14, 1176355.
View at Publisher | View at Google Scholar - Beck A. T. 2005. The current state of cognitive therapy: a 40-year retrospective. Archives of general psychiatry, 62(9), 953–959.
View at Publisher | View at Google Scholar - Clark, D. A., Beck, A. T. 2010. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends in cognitive sciences, 14(9), 418–424.
View at Publisher | View at Google Scholar - van Bentum, J. S., van Bronswijk, S. C., Sijbrandij, M., Lemmens, L., Peeters, F., Drukker, M., Huibers, M. 2021. Cognitive therapy and interpersonal psychotherapy reduce suicidal ideation independent from their effect on depression. Depression and anxiety, 38(9), 940–949.
View at Publisher | View at Google Scholar - Rachman S. 2015. The evolution of behaviour therapy and cognitive behaviour therapy. Behaviour research and therapy, 64, 1–8.
View at Publisher | View at Google Scholar - Hayes, S. C., Levin, M. E., Plumb-Vilardaga, J., Villatte, J. L., Pistorello, J. 2013. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behavior therapy, 44(2), 180–198.
View at Publisher | View at Google Scholar - Zhang, Z., Zhang, L., Zhang, G., Jin, J., Zheng, Z. 2018. The effect of CBT and its modifications for relapse prevention in major depressive disorder: a systematic review and meta-analysis. BMC psychiatry,18(1), 50.
View at Publisher | View at Google Scholar - Bhattacharyya, S., Dunlop, B. W., Mahmoudiandehkordi, S., Ahmed, A. T., Louie, G., Frye, M. A., Weinshilboum, R. M., Krishnan, R. R., Rush, A. J., Mayberg, H. S., Craighead, W. E., Kaddurah-Daouk, R. 2019. Pilot Study of Metabolomic Clusters as State Markers of Major Depression and Outcomes to CBT Treatment. Frontiers in neuroscience, 13, 926.
View at Publisher | View at Google Scholar - Nakagawa, A., Mitsuda, D., Sado, M., Abe, T., Fujisawa, D., Kikuchi, T., Iwashita, S., Mimura, M., Ono, Y. 2017. Effectiveness of Supplementary Cognitive-Behavioral Therapy for Pharmacotherapy-Resistant Depression: A Randomized Controlled Trial. The Journal of clinical psychiatry, 78(8), 1126–1135.
View at Publisher | View at Google Scholar - Toups, M., Carmody, T., Greer, T., Rethorst, C., Grannemann, B., Trivedi, M. H. 2017. Exercise is an effective treatment for positive valence symptoms in major depression. Journal of affective disorders, 209, 188–194.
View at Publisher | View at Google Scholar - Kandola, A., Ashdown-Franks, G., Hendrikse, J., Sabiston, C. M., Stubbs, B. 2019. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neuroscience and biobehavioral reviews, 107, 525–539.
View at Publisher | View at Google Scholar - Deslandes, A., Moraes, H., Ferreira, C., Veiga, H., Silveira, H., Mouta, R., Pompeu, F. A., Coutinho, E. S., Laks, J. 2009. Exercise and mental health: many reasons to move. Neuropsychobiology, 59(4), 191–198.
View at Publisher | View at Google Scholar - Sleiman, S. F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T., Ulja, D., Karuppagounder, S. S., Holson, E. B., Ratan, R. R., Ninan, I., Chao, M. V. 2016. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife, 5, e15092.
View at Publisher | View at Google Scholar - Daniele, T., de Bruin, P., Rios, E., de Bruin, V. 2017. Effects of exercise on depressive behavior and striatal levels of norepinephrine, serotonin and their metabolites in sleep-deprived mice. Behavioural brain research, 332, 16–22.
View at Publisher | View at Google Scholar - Gokdemir, O., Cetinkaya, C., Gumus, H., Aksu, I., Kiray, M., Ates, M., Kiray, A., Baykara, B., Baykara, B., Sisman, A. R., Uysal, N. 2020. The effect of exercise on anxiety- and depression-like behavior of aged rats. Biotechnic & histochemistry official publication of the Biological Stain Commission, 95(1), 8–17.
View at Publisher | View at Google Scholar - Lapmanee, S., Charoenphandhu, J., Teerapornpuntakit, J., Krishnamra, N., Charoenphandhu, N. 2017. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PloS one, 12(11), e0187671.
View at Publisher | View at Google Scholar - Carneiro, L. S., Mota, M. P., Vieira-Coelho, M. A., Alves, R. C., Fonseca, A. M., Vasconcelos-Raposo, J. 2017. Monoamines and cortisol as potential mediators of the relationship between exercise and depressive symptoms. European archives of psychiatry and clinical neuroscience, 267(2), 117–121.
View at Publisher | View at Google Scholar - Wang, L. R., Kim, S. H., Baek, S. S. 2019. Effects of treadmill exercise on the anxiety-like behavior through modulation of GSK3β/β-catenin signaling in the maternal separation rat pup. Journal of exercise rehabilitation, 15(2), 206–212.
View at Publisher | View at Google Scholar - Berger, T., Lee, H., Young, A. H., Aarsland, D., Thuret, S. 2020. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer's Disease. Trends in molecular medicine, 26(9), 803–818.
View at Publisher | View at Google Scholar - Lee, M. M., Reif, A., Schmitt, A. G. 2013. Major depression: a role for hippocampal neurogenesis?. Current topics in behavioral neurosciences, 14, 153–179.
View at Publisher | View at Google Scholar - Anacker, C., Hen, R. 2017. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nature reviews. Neuroscience, 18(6), 335–346.
View at Publisher | View at Google Scholar - Ströhle, A., Schmidt, D. K., Schultz, F., Fricke, N., Staden, T., Hellweg, R., Priller, J., Rapp, M. A., Rieckmann, N. 2015. Drug and Exercise Treatment of Alzheimer Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Effects on Cognition in Randomized Controlled Trials. The American journal of geriatric psychiatry, 23(12), 1234–1249.
View at Publisher | View at Google Scholar - Kouloutbani, K., Karteroliotis, K., Politis, A. 2019. The effect of physical activity on dementia, 30(2), 142–155.
View at Publisher | View at Google Scholar - Li, X. H., Zhu, H. C., Cui, X. M., Wang, W., Yang, L., Wang, L. B., Hu, N. W., & Duan, D. X. 2023. Death-associated protein kinase 1 is associated with cognitive dysfunction in major depressive disorder. Neural regeneration research, 18(8), 1795–1801.
View at Publisher | View at Google Scholar - Kerling, A., Kück, M., Tegtbur, U., Grams, L., Weber-Spickschen, S., Hanke, A., Stubbs, B., Kahl, K. G. 2017. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. Journal of affective disorders, 215, 152–155.
View at Publisher | View at Google Scholar - Power, R. A., Tansey, K. E., Buttenschøn, H. N., Cohen-Woods, S., Bigdeli, T., Hall, L. S., Kutalik, Z., Lee, S. H., Ripke, S., Steinberg, S., Teumer, A., Viktorin, A., Wray, N. R., Arolt, V., Baune, B. T., Boomsma, D. I., Børglum, A. D., Byrne, E. M., Castelao, E., Craddock, N., … Lewis, C. M. 2017. Genome-wide Association for Major Depression Through Age at Onset Stratification: Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Biological psychiatry, 81(4), 325–335.
View at Publisher | View at Google Scholar - Rethorst, C. D., South, C. C., Rush, A. J., Greer, T. L., Trivedi, M. H. 2017. Prediction of treatment outcomes to exercise in patients with nonremitted major depressive disorder. Depression and anxiety, 34(12), 1116–1122.
View at Publisher | View at Google Scholar - Leiter, O., Bernas, S. N., Seidemann, S., Overall, R. W., Horenburg, C., Kowal, S., Kempermann, G., Walker, T. L. 2019. The systemic exercise-released chemokine lymphotactin/XCL1 modulates in vitro adult hippocampal precursor cell proliferation and neuronal differentiation. Scientific reports, 9(1), 11831.
View at Publisher | View at Google Scholar - Andrade, A., Steffens, R., Vilarino, G. T., Sieczkowska, S. M., Coimbra, D. R. 2017. Does volume of physical exercise have an effect on depression in patients with fibromyalgia?. Journal of affective disorders, 208, 214–217.
View at Publisher | View at Google Scholar - Deuster, P. A., Chrousos, G. P., Luger, A., DeBolt, J. E., Bernier, L. L., Trostmann, U. H., Kyle, S. B., Montgomery, L. C., Loriaux, D. L. 1989. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism: clinical and experimental, 38(2), 141–148.
View at Publisher | View at Google Scholar - Ignácio, Z. M., da Silva, R. S., Plissari, M. E., Quevedo, J., Réus, G. Z. 2019. Physical Exercise and Neuroinflammation in Major Depressive Disorder. Molecular neurobiology, 56(12), 8323–8335.
View at Publisher | View at Google Scholar - Spielman, L. J., Little, J. P., Klegeris, A. 2016. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain research bulletin, 125, 19–29.
View at Publisher | View at Google Scholar - Kraus, C., Kadriu, B., Lanzenberger, R., Zarate, C. A., Jr, Kasper, S. 2019. Prognosis and improved outcomes in major depression: a review. Translational psychiatry, 9(1), 127.
View at Publisher | View at Google Scholar - Craighead, W. E., Dunlop, B. W. 2014. Combination psychotherapy and antidepressant medication treatment for depression: for whom, when, and how. Annual review of psychology, 65, 267–300.
View at Publisher | View at Google Scholar - Liu, L., Lv, X., Zhou, S., Liu, Q., Wang, J., Tian, H., Zhang, K., Wei, J., Wang, C., Chen, Q., Zhu, G., Wang, X., Zhang, N., Huang, Y., Si, T., Yu, X. 2021. The effect of selective serotonin reuptake inhibitors on cognitive impairment in patients with depression: A prospective, multicenter, observational study. Journal of psychiatric research, 141, 26–33.
View at Publisher | View at Google Scholar - Ling. H. W. 2021. Why Patients With Depression Do Not Improve their Symptoms When Using Anti-Depressant Medications? Intern Jour psych 6(2): 54-62.
View at Publisher | View at Google Scholar