Case Report | DOI: https://doi.org/10.58489/2836-2217/016
Clinical Case of Suspected Caecal Appendicitis Concealing Right Renal Artery Thrombosis
- Paolo Ghiringhelli 1*
- Federica Macchi 1
- Mariella Ciola 1
- Andrea Agostinelli 1
- Lorenzo Bellintani 1
- Beatrice Valvo 1
- Gaetano Emanuele Rizzo 1
- Michela Provisione 1
- Michela Zaza 1
- Alessandro Diana 1
- Patrizia Marinoni 1
- Luigi Pelucco 1
- Erica Pagliaro 1
- Anna Maria Socrate 2
- General Internal Medicinand Unit, Busto Arsizio Hospital, ASST Valle Olona Data Manager Lara Carabelli.
- Director of the Nephro-vascular Department, Valle Olona social health company. Director of the Vascular Surgery, Busto Arsizio Hospital, ASST Valle Olona
*Corresponding Author: Paolo Ghiringhelli
Citation: Paolo Ghiringhelli, Federica Macchi, Mariella Ciola, Andrea Agostinelli, Lorenzo Bellintani, Beatrice Valvo, Gaetano Emanuele Rizzo, Michela Provisione, Michela Zaza, Alessandro Diana, Patrizia Marinoni, Luigi Pelucco, Erica Pagliaro, Anna Maria Socrate. (2024). Clinical Case of Suspected Caecal Appendicitis Concealing Right Renal Artery Thrombosis. Journal of Clinical Case Reports and Trails. 3(1). DOI: 10.58489/2836-2217/016
Copyright: © 2024 Paolo Ghiringhelli, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 25 September 2023 | Accepted: 02 February 2024 | Published: 09 February 2024
Keywords: medical history, right side pain and right iliac fossa, renal infarction, antiphospholipid antibodies
Abstract
We describe an Equadorean patient, 48 years old, who registered to the Emergency Room with pain, fever, leukocytosis, and c-reactive protein in the right side and right iliac fossa and was admitted for suspected acute appendicopathy. Subjected to abdominal CT scan, the presence of a right renal infarct area confirmed by angiographic study is detected. Admitted to Vascular Surgery, he was transferred to Medicine and detected to be double positive for phospholipid antibodies, concomitantly elevated triglyceride values were found. The patient, treated during hospitalization with heparin, was discharged with Warfarin-based oral anticoagulant therapy being contraindicated with direct coagulation inhibitors in the presence of antiphospholipid antibodies.
Introduction
Why do we describe the case?
- Because of the unexpected onset of uncommon vascular disease
- Because of the differential diagnosis that had to be made in order to identify the pain in the iliac fossa-right side
- Because of the importance of the family history also in order to identify correct diagnostic-therapeutic plans
- Because of the isolated phospholipid antibody positivity in a body that otherwise appeared to be in excellent general condition
Clinical case
A 48-year-old Ecuadorean man works as a delivery man. He registered to the Emergency Room because of abdominal pain in the right side and in the right iliac fossa.
He reports positive paternal family history of unspecified thrombotic events. No home therapy, denies drug allergies.
He underwent surgery in neonatal period to correct omphalocele
Obesity class I (BMI 31.6 by weight 90 kg).
Upcoming Pathological History:
On the 16th, April 2023 pain in right side appears followed by macroscopic hematuria and fever. Therefore, on the 19th April he was sent to the ER by the GP. Thanks to a CT abdomen with contrast agent, a right renal infarct with focal filling defect at the level of the cortical branch of the right renal artery in the presence of double renal artery bilaterally was detected. -Blood tests on admission GB 12.53, Hb 15, PLT 236.000; PCR 5.57, creatinine 0.99, electrolytes within limits. The vascular surgeon indicated that the patient should undergo right renal angiography and possible thromboaspiration/stenting of the renal artery. On the 19th April 2023, he underwent right renal artery thrombo-aspiration surgery via percutaneous right femoral access in the angiography room without creating significant complications.
He was subsequently transferred to Internal Medicine to undergo appropriate differential diagnosis.
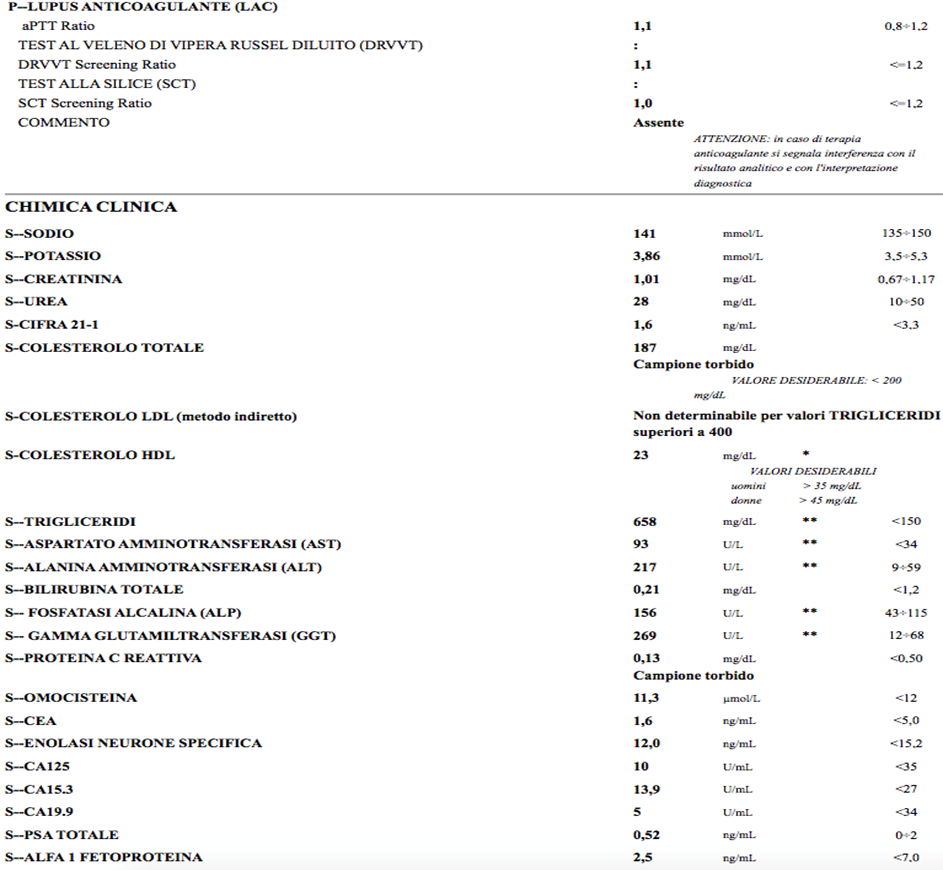
The progress was free of hemorrhagic, infectious complications; renal function remained stable and normal Tests were performed to search the presence of hereditary or acquired thrombophilic status, lipid profile, with severe hypertriglyceridemia found.
The supra-aortic arterial district was studied with echo Doppler, which showed no plaques. CT angiography of the abdomen performed at the time of diagnosis had ruled out the presence of plaques at the level of the large abdominal vessels. A Holter ECG and echocardiogram and lower extremity arterial Doppler echo were requested. All of the above investigations led to no significant pathological findings. The patient was discharged with an indication to take ASA 100 mg/die together with CLEXANE 8000 IU 1 FL SC every 12 hours until the INR stabilized between 2 and 3 with warfarin. Also recommended: atorvastatin 20 mg in the evening, folin 5 mg for the presence of modest increase in homocysteinemia.
Re-evaluated 3 months later, positivity of IGG 34.4 cardiolipin antibodies, IGG 36 beta two microglobulin was confirmed; IGM normal; LAC absent; presence of heterozygosity of MTHFR: finding of hypertriglyceridemia 658, total cholesterol 187 (already on atorvastatin 20 mg therapy since dmission) LDL not calculable
-VSX echocardiogram Slightly hypertrophied Inter Ventricular septum 11, Posterior wall (PP) 11 Transverse diastolic diameter (DTD 50 mmm) normal kinesis aortic root: slightly dilated with bulb 24 mm tubular tract 39, arch 25 mm, aortic valve not well visualized, probably tricuspid -ECG holter diffuse alterations in ventricular repolarization
Conclusions: thrombosis of a collateral branch of the right renal artery, conditioning renal infarction, in patient with double positivity for anti-phospholipid antibodies (Aanti cardiolipin IgG and Anti B2 Glycoprotein 1) IgG, with absent LAC; hypertriglyceridemia; hypertension likely misdiagnosed; obesity grade I.
In view of the currently high-risk profile, we recommended standard-range Coumadin therapy (INR 2-3) together with aspirin 75 mg/die on a full stomach along with a hypolipid diet for hypertriglyceridemia)
Continuation of atorvastatin 20 mg by adding polyunsaturated fatty acid ethyl esters 1gr every 8 hours was indicated.
LDHs were elevated: 559 U/L (vn maximum 220),
Discussion
Renal infarction is rare (1-7). In a study of 14,411 autopsies published in 1940, the incidence of renal infarction was 1.4% (6). In a subsequent series of nearly 250,000 patients seen in an Emergency Room over four years, only 17 (0.007%) were diagnosed with acute renal infarction (7). Depending on the severity, it can lead to nephrovascular hypertension, chronic kidney disease, and end-stage renal disease. The frequency of renal infarction is probably higher than reported in the above studies because the clinical diagnosis of renal infarction is often missed or delayed because patients present with abdominal or side pain that mimics other more common conditions, such as nephrolithiasis and pyelonephritis or, as in our patient, the pain being on the right side, was initially interpreted as an attack of vermiform appendicitis cecum. The two main causes of renal infarction are thromboemboli and in situ thrombosis. Thromboemboli usually originate from a thrombus in the heart or aorta, and in situ thrombosis is usually due to an underlying hypercoagulable condition or injury or dissection of a renal artery. Both thromboemboli and in situ thrombi can cause complete occlusion of the main renal artery or smaller segmental branch arteries (1,7). Atheroemboli often lead to secondary ischemic atrophy rather than renal infarction because of their nondistensibility, irregular shape, and small size. Atheroemboli often lead to incomplete arterial occlusion of the most distal vessels.
The main etiologies
The main etiologies of renal infarction include cardioembolic disease, renal artery injury (most commonly due to artery dissection) and hypercoagulation states (1,3,5,8-10):
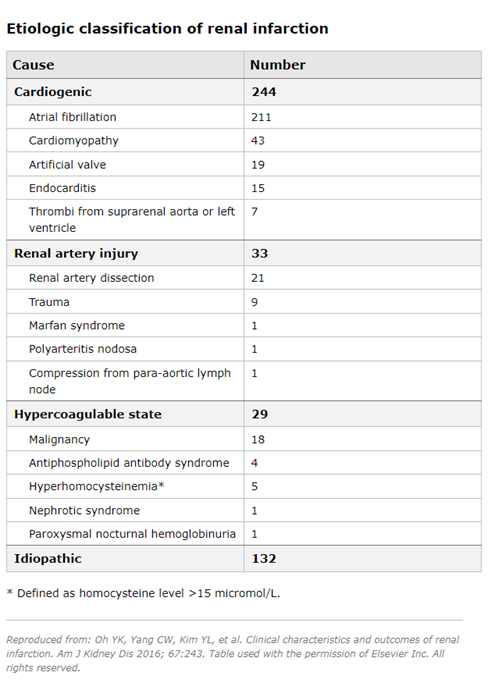
Cardioembolic disease - In a series of 438 patients with renal infarction diagnosed between 1993 and 2013, 244 (55.7%) patients had cardioembolic renal infarction and 211 patients had atrial fibrillation (9). Underlying heart diseases in the cardioembolic group included cardiomyopathy, endocarditis and artificial valve thrombi. Renal infarction is more common among patients with atrial fibrillation. In a cohort study that included nearly 30,000 patients with atrial fibrillation, compared with the general Danish population, males and females with atrial fibrillation had a higher relative risk of thromboembolic events (4 and 5.7, respectively) (11). Among the 621 individuals with arterial thromboembolism, 2% of presentations involved the renal artery. Note that renal infarction may be the first manifestation of atrial fibrillation, and in established cases of atrial fibrillation, many patients who developed renal infarction were not in therapeutic ranges with warfarin (8).
Renal artery injury - In the above case series, 33 patients (7.5%) had an underlying renal artery injury (9). Other less common etiologies reported elsewhere associated with renal artery injury include fibromuscular dysplasia (56), Ehlers-Danlos syndrome (1), segmental arterial mediolysis (SAM) (12), renal artery occlusion following aortic or renal endovascular surgery (7,13-17), and cocaine use (18). Hypercoagulability status - In the above case series, 29 patients (6.6%) had hypercoagulability status due to hereditary thrombophilia in six, hyperhomocysteinemia in four, antiphospholipid syndrome in four and nephrotic syndrome in one (9). Renal infarction has also been reported in association with COVID-19, both in native (19-23) and transplanted kidneys (24). This may be due to endothelial dysfunction and activation of the coagulation cascade resulting from the inflammatory response during COVID-19 (21). Renal infarction can also be observed among women using the oral contraceptive pill (25-27).
In the above case series, no cause could be identified in 132 patients (30.1%) (9). In another study of 27 consecutive patients with nontraumatic acute renal infarction, 16 (59%) had no recognizable structural or arrhythmic heart disease and were classified as idiopathic (28).
Our patient was also studied for inherited and acquired thrombotic diathesis, and only anti-cardiolipin IgG and Beta two microglobulin 1 IgG antibodies were pathological. However, the patient had comorbidities that may have facilitated the thrombophilic diathesis: hypertriglyceridemia, at first hypothesis endogenous, obesity, and sedentary lifestyle. Probably of minor importance is MTHFR heterozygosity given the modest increase in circulating homocysteine.
In a case series of 438 patients with acute renal infarction, the following demographic characteristics were found (9):
The median age varied according to the underlying etiology: cardiogenic (65 years), from renal artery injury (43 years), from hypercoagulable state (62 years), and idiopathic (49.5 years).
Patients in the cardiogenic group had a more frequent history of hypertension, diabetes mellitus, cardiovascular disease (CVD), heart valve disease, and atrial fibrillation than their counterparts in the other three groups.
Patients with acute renal infarction typically complain of acute onset of flank or abdominal pain, often accompanied by nausea, vomiting, and occasionally fever (1-4,7-9,29). In the above case series of 438 patients, flank pain was present in 50%, abdominal pain in 53%, nausea in 16.9%, vomiting in 13%, and fever in 10% (1). Bilateral renal involvement was present in 16.9% (9).
These findings may be accompanied by acute elevation of blood pressure, presumably mediated by increased renin release (1,7,30,31). Signs of extrarenal embolization (such as focal neurological deficits and mesenteric and limb ischemia) can be observed. Some patients may be asymptomatic, and their renal infarctions are brought to clinical attention by an incidental finding on an imaging study performed for an unrelated condition (7).
Related laboratory manifestations
The frequency of laboratory pathological findings characteristic of renal thrombosis were derived from the case series of 438 patients mentioned above (9):
-Hematuria was present in 32% of patients, our patient had it a few hours after admission, and proteinuria in 12%.
-Mean serum creatinine concentration was 1.0 mg/dL (84.0 micromol/L), range 0.4 to 5.6 mg/dL. In our patient the creatinine values were normal, in fact the embolism was unilateral.
In another study, the reduction in renal function was more pronounced in patients with bilateral disease or a large unilateral embolus (1).
-In the above case histories (9), the serum concentration of lactate dehydrogenase (LDH) was increased, similar to our patient's case, (mean 656 international units/L (range 152 to 7660)). Some smaller studies had higher mean serum levels of LDH from 1100 to 1570 international units/L (3,28,30).
In the appropriate clinical setting, an elevated serum LDH level (often two to four times the upper limit of normal) with little or no increase in serum aminotransferases is strongly suggestive of renal infarction (1-3,28,30,33). LDH elevation can also be seen in other conditions that are usually easily distinguishable from renal infarction, including late myocardial infarction, hemolysis, and kidney transplant rejection (33). The LDH figure in our patient was somewhat marred by the presence also of significant dyslipidemia with elevated GGT for very likely steatohepatitis.
-Other evidence demonstrated the presence of a mildly elevated white blood cell count (mean 11,000/microL) in our case 12530 mcl with 9700 neutrophil mcl and an increase in serum C-reactive protein, which was 5.57 mg/dl (vn <0>
Diagnosis
Since the presenting symptoms of renal infarction are not frankly pathognomonic, the time between presentation and diagnosis is often more than two days, with less than 50% of patients diagnosed promptly (3,31,34). Our patient was fortunate enough to have the infarct on the right side, and the pain led to the suspicion of acute appendicopathy, which prompted the GP in the ER to perform a spiral CT scan that revealed the patient's real problem.
In patients who are at risk of systemic embolization and have symptoms suggestive of renal infarction, we should request the following laboratory tests (2): ●Complete blood count with differential ●Serum creatinine and lactate dehydrogenase (LDH) ●Urinal urinalysis and urinoculture ●Electrocardiogram to evaluate atrial fibrillation Noncontrast computed tomography (CT) is usually the preferred initial test for flank pain because it is the gold standard for the diagnosis of kidney and ureteral stones, which are much more common than renal infarction (3). Our patient had normal renal function, which had allowed a CT scan with contrast medium to be performed (32).
Among patients who have a clinical presentation compatible with renal infarction rather than nephrolithiasis, a CT scan with contrast medium should be performed rather than a CT scan without contrast medium. The classic finding is a wedge-shaped perfusion defect as observed in our patient. Magnetic resonance imaging (MRI) with gadolinium is an alternative to CT (35), although the use of gadolinium should be based on the patient's renal function,
Radioisotope scintigraphy may show segmental or generalized decrease in renal perfusion. Although radioisotope scans were commonly used in the past, their use has been largely supplanted by new imaging techniques. Ultrasound is much less sensitive and therefore much less useful in urgency for this type of problem.
The sensitivity of the above imaging techniques was evaluated in a case series of 44 patients with atrial fibrillation and a diagnosis of embolic renal infarction (3). Sensitivity was 97% (36/37) with radioisotope renal scan, 80% (12/15) with contrast-enhanced CT scan, and only 11% with renal ultrasound (3).
Differential diagnosis
The two conditions that most closely mimic the clinical presentation of acute renal infarction are renal colic (flank pain and hematuria) and acute pyelonephritis (flank pain and fever). Our patient had pain referred to the appendicular area and was at first interpreted as acute appendicitis. In a report of 14 patients, for example, eight had an inpatient diagnosis of nephrolithiasis (36). However, neither nephrolithiasis nor pyelonephritis is associated with increased serum LDH.
Renal infarction often presents with a predominance of hematuria, but may also be associated with pyuria, although usually without bacteriuria. Nephrolithiasis may present with any of these findings on urinalysis.
Other conditions that may mimic some of the features of renal infarction include mesenteric ischemia and other causes of abdominal pain, such as cholecystitis and pancreatitis. A history of atrial fibrillation or recent intra-arterial manipulation increases the likelihood of renal infarction or mesenteric ischemia.
Therapeutic management
Assess the probability of benefit from revascularization -
One must assess the time since the onset of ischemia (determined by the duration of symptoms and signs), the size of the renal parenchyma threatened by the infarct, renal function (i.e., estimated glomerular filtration rate (eGFR)), and whether the renal vessel is completely or partially occluded (using information obtained from computed tomography angiography (CTA) in most patients).
Based on these factors, the following patients are, in general, more likely to benefit from revascularization than others are patients with the following clinical conditions:
● Complete occlusion of the main renal artery (or major segmental branch) lasting <6>
● Partial main or major segmental renal artery occlusion lasting <24>
● Partial main or major segmental renal artery occlusion lasting 24 hours or more in the presence of significant renal insufficiency, new or worsened hypertension, or symptoms such as flank pain, hematuria, and fever.
● Patients who have arterial dissection as a cause of renal infarction.
We do not perform a CTA when the initial diagnostic CT scan demonstrates an atrophic kidney or dense wedge-shaped scar, suggesting a remote event without viable tissue. Such a kidney may also have a well-developed collateral circulation, which will reduce the potential benefit from correcting the renal artery occlusion. Under these circumstances, revascularization may be of negligible benefit.
Evaluation of the probability of benefit from revascularization is based on the following factors:
● Vessel type-The risk of parenchymal damage depends on the type of vessel involved (main, segmental, or subsegmental artery) (37). Occlusion of the main renal artery threatens loss of function of the entire kidney. Occlusion of segmental arteries, as in the case of our patient, generally leads to hypoperfusion of large portions of the kidney, which can be significant in patients with only one kidney or in patients with marked impairment of renal function. Occlusion of a subsegmental artery generally leads to a wedge-shaped defect in the parenchyma.
● Time from the onset of ischemia-The time from the onset of ischemia also affects the probability that parenchymal damage may be recoverable. Renal parenchyma is less likely to recover if the duration of ischemia is long. Symptoms such as acute flank pain, nausea, vomiting, and acute rise in blood pressure often suggest a recent event (usually less than a week before). On the other hand, a small kidney on the affected side suggests that there has been prolonged ischemia. Often, patients incidentally present with wedge-shaped parenchymal perfusion defects on abdominal imaging performed for another unrelated indication. In such situations, the age of the infarct is indeterminate and should be treated as remote unless symptomatic.
● Renal function impairment-The degree of renal function impairment may depend on the extent of parenchymal damage and the function of the contralateral (uninvolved) kidney. In patients with normal renal function, even unilateral complete renal artery occlusion may not affect overall renal function. However, this is not the case for patients who have acute kidney injury (AKI) or have preexisting chronic kidney disease. There may also be marked loss of renal function in situations where there is occlusion of bilateral renal arteries or occlusion of the renal artery that perfuses a single functioning kidney.
Assess a state of hypercoagulability or other predisposing condition -
In the absence of a preexisting diagnosis, all patients with renal infarction should be evaluated for atrial fibrillation. In addition, we evaluate all patients for an underlying hypercoagulable state. Details regarding the diagnosis of atrial fibrillation and hypercoagulable state are presented elsewhere.
THERAPY
The optimal treatment for renal infarction due to thromboemboli, in situ thrombosis, or renal artery dissection is uncertain given the absence of comparative studies. Approaches reported include anticoagulation, percutaneous endovascular therapy (thrombolysis, thrombectomy with or without angioplasty or stent placement), and open surgery. Surgery is an option for patients with renal infarction resulting from traumatic renal artery occlusion or aortic dissection extending into the renal artery (38-41).
Main and major segmental renal artery involvement -
It is necessary to restore perfusion in patients with main renal artery occlusion (37). Intervention may be considered in patients with first-order branch occlusion in the context of a solitary kidney or with significantly reduced renal function (e.g., estimated glomerular filtration rate (eGFR) <50>
Patients who could benefit from revascularization -
Patients who might benefit from revascularization should be referred immediately to a vascular interventional radiology or vascular surgery service for percutaneous endovascular therapy (PET) (38,42). PET may include local thrombolysis, thrombectomy, angioplasty, and stent placement (43-45). The choice of the optimal endovascular procedure is usually left to the interventionalist or surgeon. If significant residual vascular abnormalities are observed after thrombectomy, the vessel is treated with angioplasty with or without stent placement. If PET is not available, such patients should undergo systemic thrombolysis (46). Details regarding the assessment of whether or not a patient will benefit from revascularization are discussed above. Intraprocedural heparin is usually administered during thrombolysis and discontinued shortly after the procedure. Patients who have an underlying vascular abnormality or who have undergone angioplasty or stent placement should be treated with aspirin 81 mg and clopidogrel 75 mg for 3 to 6 months after the procedure followed thereafter by aspirin alone. In addition, we treat patients who have an underlying hypercoagulable state or atrial fibrillation who have a stent placed with anticoagulants and low-dose aspirin (e.g., 81 mg daily).
Where vascular surgery services are not available, systemic fibrinolytic therapy may be used, although data supporting this approach are scarce (47). The risks of significant bleeding are higher with systemic thrombolytic therapy than with local thrombolysis in the context of PET.
The maximum duration of complete renal artery occlusion beyond which thrombolysis would no longer be useful is unknown. One study reported little benefit after 90 minutes, while other studies found some benefit up to several days later (36,42-44,48,49). Delayed therapy is likely to be effective in patients with partial occlusion and patients with thrombotic occlusion (38).
In our patient's case, arrival in the emergency room was 4 days after the onset of symptoms and still allowed aspiration of the thrombus. It is unclear what the benefit will be on the infarcted renal area, but the pain and macrohematuria quickly disappeared.
PET leads to successful reperfusion in most patients with renal infarction without significant complications associated with therapy; however, renal outcomes are improved in only a few patients (36,43,44,48,50-54). Observational studies of intra-arterial thrombolytic therapy illustrate the range of results: ●One study included 14 patients with acute embolic renal artery occlusion who were treated with intra-arterial thrombolysis using urokinase, streptokinase, or recombinant tissue plasminogen activator (36,50). The diagnosis of renal infarction was made within 36 hours in only eight patients; the delay in diagnosis in the remaining patients was up to eight days after the onset of symptoms. Complete renal artery occlusion was observed in five patients and partial occlusion was observed in eight patients (main renal artery in four and segmental arteries in four); one patient had bilateral renal artery occlusion. Revascularization was successful in 13 patients. Patients with complete main renal artery occlusion had no improvement in renal function after revascularization. In contrast, stabilization or slight improvement of renal function was observed in patients with partial occlusion or complete occlusion of segmental branches. Macroscopic hematuria and postprocedural hematoma were observed in one patient each, neither of whom required surgery.
•In a series of 10 patients with main renal artery or segmental branch occlusion (three thrombotic, two embolic, one associated with aortic occlusion, and the rest as a complication of renal artery angioplasty), all received intra-arterial thrombolysis with urokinase or streptokinase; Percutaneous transluminal angioplasty was performed in five patients (48). Therapy was started within 24 hours in only three patients, and the rest were treated up to five weeks after the onset of symptoms. Successful revascularization confirmed by arteriography was achieved in 7 of 10 patients. Of these seven patients, recovery of kidney function occurred in three patients who had been treated at one, two and six days after the onset of symptoms. As a complication of thrombolytic therapy, a patient with aortic occlusion developed an embolic infarction of the superior mesenteric artery that necessitated colon resection.
●In a retrospective study of 42 patients, 13 patients were treated with PET and the remaining with conservative therapy (42). Main renal artery involvement was observed in 85% of patients treated with PET compared with 20% treated with conservative care. Partial or complete restoration of flow was confirmed in all patients treated with PET. At a median follow-up of 30 months, mean creatinine clearance (CrCl) in the PET group had decreased from 74 to 55 ml/min. Two patients required permanent dialysis despite PET scan, both with complete renal artery occlusion of the transplanted kidney. In the conservative care group, the median follow-up was 13 months, and mean CrCl decreased from 66 to 60 ml/min. No procedural complications were reported in this study.
Patients unlikely to benefit from revascularization - In patients with main renal artery occlusion but unlikely to benefit from revascularization (see "Assessing the likelihood of benefit from revascularization" above), our approach depends on our assessment of how much time has passed since the infarction (Algorithm 1): ● Patients with a remote infarct - Patients who are asymptomatic (i.e., without acute symptoms such as flank pain) and who have a small atrophic kidney or a wedge-shaped parenchymal perfusion defect on the affected side may have a remote infarct. Such patients should be evaluated for atheroembolic risk factors such as atrial fibrillation (see "Atrial fibrillation: overview and management of new-onset atrial fibrillation"). If this evaluation is negative, an evaluation to look for a hypercoagulable state should be completed (see 'Evaluation for a hypercoagulable state or other predisposing condition' above). If, after this evaluation, there is no indication for anticoagulant therapy, we manage these patients with aspirin therapy. (See “Accidentally detected renal infarction or atrophic kidney” below.) ● Patients with more recent infarction - In patients who are symptomatic (or recently symptomatic) and whose imaging suggests more recent infarction, we initiate anticoagulation. The duration of anticoagulation depends on the assessment of a hypercoagulable state and other risk factors for arterial embolization.
In patients without a predisposing underlying condition such as atrial fibrillation, they anticoagulate for six months, although data to support this approach are generally lacking. In the absence of phospholipid antibodies, direct oral anticoagulants could be used, preferably apixaban because of its lower renal clearance. The use of warfarin as an alternative anticoagulant is mandated in the presence of anti-phospholipid antibodies given the known lower efficacy of direct inhibitors in the presence of anti-phospholipid antibodies. For patients treated with warfarin, the goal is to titrate the drug while maintaining the INR between 2 and 3, except if the renal infarction occurred in a patient already on warfarin therapy, in which case we increase the goal to 2.5-3.5. (55)
Hypertensive crisis often appears in the course of renal thrombosis. In the absence of acute kidney injury (AKI) or hyperkalemia, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are used. In those with AKI or hyperkalemia, treatment of hypertension is no different from patients without renal infarction. (7)
Renal prognosis with anticoagulation has generally been favorable, but there are no reports comparing outcomes with untreated patients (9,30,31,55). After completion of six months of anticoagulant therapy, we start low-dose aspirin for life (e.g., 81 mg daily).
Minor segmental vessel involvement-Patients with minor segmental vessel involvement resulting from dissection or fibromuscular dysplasia (FMD) that is recent should be referred to interventional vascular radiology or vascular surgery to consider PET (Algorithm 1) (45).
The choice of anticoagulant and its titration are identical for patients with main renal artery occlusion who are unlikely to benefit from revascularization. Patients not receiving anticoagulants should start with low-dose aspirin as reported above. Patients with underlying hypercoagulable state or atrial fibrillation may require lifelong anticoagulants.
In our patient's case, it is mandatory to subject the patient to careful and individualized nutritional and drug therapy to normalize dyslipidemia and in particular hypertriglyceridemia that contributes to the prothrombotic state. Indeed, it has been known for many years that hypertriglyceridemia is an independent factor associated with increased cardiovascular risk (57, 58).
References
- Bourgault, M., Grimbert, P., Verret, C., Pourrat, J., Herody, M., Halimi, J. M., ... & Audard, V. (2013). Acute renal infarction: a case series. Clinical journal of the American Society of Nephrology: CJASN, 8(3), 392.
View at Publisher | View at Google Scholar - Domanovits, H., Paulis, M., Nikfardjam, M., Meron, G., Kürkciyan, I., Bankier, A. A., & Laggner, A. N. (1999). Acute renal infarction: clinical characteristics of 17 patients. Medicine, 78(6), 386-394.
View at Publisher | View at Google Scholar - Hazanov, N., Somin, M., Attali, M., Beilinson, N., Thaler, M., Mouallem, M., ... & Malnick, S. (2004). Acute renal embolism: forty-four cases of renal infarction in patients with atrial fibrillation. Medicine, 83(5), 292-299.
View at Publisher | View at Google Scholar - CHU, P. L., WEI, Y. F., HUANG, J. W., CHEN, S. I., CHU, T. S., & WU, K. D. (2006). Clinical characteristics of patients with segmental renal infarction. Nephrology, 11(4), 336-340.
View at Publisher | View at Google Scholar - Antopolsky, M., Simanovsky, N., Stalnikowicz, R., Salameh, S., & Hiller, N. (2012). Renal infarction in the ED: 10-year experience and review of the literature. The American journal of emergency medicine, 30(7), 1055-1060.
View at Publisher | View at Google Scholar - HOXIE, H. J., & COGGIN, C. B. (1940). Renal infarction: statistical study of two hundred and five cases and detailed report of an unusual case. Archives of Internal Medicine, 65(3), 587-594.
View at Publisher | View at Google Scholar - Paris, B., Bobrie, G., Rossignol, P., Le Coz, S., Chedid, A., & Plouin, P. F. (2006). Blood pressure and renal outcomes in patients with kidney infarction and hypertension. Journal of hypertension, 24(8), 1649-1654.
View at Publisher | View at Google Scholar - Caravaca-Fontán, F., Saico, S. P., Trivino, S. E., Álvarez, C. G., Couto, A. G., de las Heras, I. P., & Liano, F. (2016). Acute renal infarction: Clinical characteristics and prognostic factors. Nefrología (English Edition), 36(2), 141-148.
View at Publisher | View at Google Scholar - Oh, Y. K., Yang, C. W., Kim, Y. L., Kang, S. W., Park, C. W., Kim, Y. S., ... & Lim, C. S. (2016). Clinical characteristics and outcomes of renal infarction. American Journal of Kidney Diseases, 67(2), 243-250.
View at Publisher | View at Google Scholar - Faucon, A. L., Bobrie, G., Jannot, A. S., Azarine, A., Plouin, P. F., Azizi, M., & Amar, L. (2018). Cause of renal infarction: a retrospective analysis of 186 consecutive cases. Journal of Hypertension, 36(3), 634-640.
View at Publisher | View at Google Scholar - Frost, L., Engholm, G., Johnsen, S., Møller, H., Henneberg, E. W., & Husted, S. (2001). Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Archives of internal medicine, 161(2), 272-276.
View at Publisher | View at Google Scholar - Filippone, E. J., Foy, A., Galanis, T., Pokuah, M., Newman, E., Lallas, C. D., ... & Farber, J. L. (2011). Segmental arterial mediolysis: report of 2 cases and review of the literature. American journal of kidney diseases, 58(6), 981-987.
View at Publisher | View at Google Scholar - Cosby, R. L., Miller, P. D., & Schrier, R. W. (1986). Traumatic renal artery thrombosis. The American journal of medicine, 81(5), 890-894.
View at Publisher | View at Google Scholar - Krämer, S. C., Seifarth, H., Pamler, R., Fleiter, T., Bühring, J., Sunder-Plassmann, L., ... & Görich, J. (2002). Renal infarction following endovascular aortic aneurysm repair: incidence and clinical consequences. Journal of Endovascular Therapy, 9(1), 98-102.
View at Publisher | View at Google Scholar - Ivanovic, V., McKusick, M. A., Johnson III, C. M., Sabater, E. A., Andrews, J. C., Breen, J. F., ... & Stanson, A. W. (2003). Renal artery stent placement: complications at a single tertiary care center. Journal of vascular and interventional radiology, 14(2), 217-225.
View at Publisher | View at Google Scholar - Böckler, D., Krauss, M., Mannsmann, U., Halawa, M., Lange, R., Probst, T., & Raithel, D. (2003). Incidence of renal infarctions after endovascular AAA repair: relationship to infrarenal versus suprarenal fixation. Journal of Endovascular Therapy, 10(6), 1054-1060.
View at Publisher | View at Google Scholar - Stawicki, S. P., Rosenfeld, J. C., Weger, N., Fields, E. L., & Balshi, J. D. (2006). Spontaneous renal artery dissection: three cases and clinical algorithms. Journal of human hypertension, 20(9), 710-718.
View at Publisher | View at Google Scholar - Bemanian, S., Motallebi, M., & Nosrati, S. M. (2005). Cocaine-induced renal infarction: report of a case and review of the literature. BMC nephrology, 6(1), 1-6.
View at Publisher | View at Google Scholar - Post, A., den Deurwaarder, E. S., Bakker, S. J., de Haas, R. J., van Meurs, M., Gansevoort, R. T., & Berger, S. P. (2020). Kidney infarction in patients with COVID-19. American Journal of Kidney Diseases, 76(3), 431-435.
View at Publisher | View at Google Scholar - Añazco, P. H., Balta, F. M., & Córdova-Cueva, L. (2021). Bilateral renal infarction in a patient with severe COVID-19 infection. Brazilian Journal of Nephrology, 43, 127-131.
View at Publisher | View at Google Scholar - Guillet, H., Gallet, R., Pham, V., D’Humières, T., Huguet, R., Lim, P., ... & Khellaf, M. (2021). Clinical spectrum of ischaemic arterial diseases associated with COVID-19: a series of four illustrative cases. European Heart Journal-Case Reports, 5(1), ytaa488.
View at Publisher | View at Google Scholar - Topel, Ç., Yıldırım, C., Yavaş, M. A., & Göde, S. (2021). Aortic floating thrombi with lower limb ischemia and renal infarct in COVID-19: A remote thromboembolic complication. Turk Kardiyoloji Dernegi Arsivi, 49(3), 233.
View at Publisher | View at Google Scholar - Murray, N. P., Fuentealba, C., Reyes, E., & Salazar, A. (2021). Renal infarction associated with asymptomatic Covid-19 infection. Hematology, Transfusion and Cell Therapy, 43, 353-356.
View at Publisher | View at Google Scholar - Webb, C., Davidson, B., Jones, E. S., Wearne, N., Chetty, D. R., Blom, D., & Barday, Z. (2021). COVID-19–Associated Graft Loss From Renal Infarction in a Kidney Transplant Recipient. Kidney International Reports, 6(4), 1166-1169.
View at Publisher | View at Google Scholar - Bhargava, A., Chopra, A., Bernabela, L., & Chopra, T. (2013). Oral contraceptive causing renal artery thrombosis. Case Reports, 2013, bcr2012008055.
View at Publisher | View at Google Scholar - Golbus, S. M., Swerdlin, A. R., Mitas, J. A., Rowley, W. R., & James, D. R. (1979). Renal artery thrombosis in a young woman taking oral contraceptives. Annals of Internal Medicine, 90(6), 939-940.
View at Publisher | View at Google Scholar - Slick, G. L., Schnetzler, D. E., & Kaloyanides, G. J. (1975). Hypertension, renal vein thrombosis and renal failure (occurring in a patient on an oral contraceptive agent). Clinical Nephrology, 3(2), 70-74.
View at Publisher | View at Google Scholar - Bolderman, R., Oyen, R., Verrijcken, A., Knockaert, D., & Vanderschueren, S. (2006). Idiopathic renal infarction. The American journal of medicine, 119(4), 356-e9.
View at Publisher | View at Google Scholar - LUMERMAN, J. H., HOM, D., EILEY, D., & SMITH, A. D. (1999). Heightened suspicion and rapid evaluation with CT for early diagnosis of partial renal infarction. Journal of endourology, 13(3), 209-214.
View at Publisher | View at Google Scholar - Korzets, Z. E., Plotkin, E., Bernheim, J., & Zissin, R. (2002). The clinical spectrum of acute renal infarction. IMAJ-RAMAT GAN-, 4(10), 781-784.
View at Publisher | View at Google Scholar - LESSMAN, R. K., JOHNSON, S. F., COBURN, J. W., & KAUFMAN, J. J. (1978). Renal artery embolism: clinical features and long-term follow-up of 17 cases. Annals of internal medicine, 89(4), 477-482.
View at Publisher | View at Google Scholar - London, I. L., Hoffsten, P., Perkoff, G. T., & Pennington, T. G. (1968). Renal infarction: Elevation of serum and urinary lactic dehydrogenase (LDH). Archives of Internal Medicine, 121(1), 87-90.
View at Publisher | View at Google Scholar - Winzelberg, G. G., Hull, J. D., Agar, J. W., Rose, B. D., & Pletka, P. G. (1979). Elevation of serum lactate dehydrogenase levels in renal infarction. Jama, 242(3), 268-269.
View at Publisher | View at Google Scholar - Tsai, S. H., Chu, S. J., Chen, S. J., Fan, Y. M., Chang, W. C., Wu, C. P., & Hsu, C. W. (2007). Acute renal infarction: a 10‐year experience. International journal of clinical practice, 61(1), 62-67.
View at Publisher | View at Google Scholar - Kim, S. H., Park, J. H., Han, J. K., Han, M. C., Kim, S., & Lee, J. S. (1992). Infarction of the kidney: role of contrast enhanced MRI. Journal of computer assisted tomography, 16(6), 924-928.
View at Publisher | View at Google Scholar - Blum, U., Billmann, P., Krause, T., Gabelmann, A., Keller, E., Moser, E., & Langer, M. (1993). Effect of local low-dose thrombolysis on clinical outcome in acute embolic renal artery occlusion. Radiology, 189(2), 549-554.
View at Publisher | View at Google Scholar - Koivuviita, N., Tertti, R., Heiro, M., Manner, I., & Metsärinne, K. (2014). Thromboembolism as a cause of renal artery occlusion and acute kidney injury: the recovery of kidney function after two weeks. Case reports in nephrology and urology, 4(1), 82-87.
View at Publisher | View at Google Scholar - Ouriel, K., Andrus, C. H., Ricotta, J. J., DeWeese, J. A., & Green, R. M. (1987). Acute renal artery occlusion: when is revascularization justified?. Journal of vascular surgery, 5(2), 348-355.
View at Publisher | View at Google Scholar - Haas, C. A., Dinchman, K. H., Nasrallah, P. F., & Spirnak, J. P. (1998). Traumatic renal artery occlusion: a 15-year review. Journal of Trauma and Acute Care Surgery, 45(3), 557-561.
View at Publisher | View at Google Scholar - Haas, C. A., & Spirnak, J. P. (1998). Traumatic renal artery occlusion: a review of the literature. Techniques in urology, 4(1), 1-11.
View at Publisher | View at Google Scholar - Bouttier, S., Valverde, J. P., Lacombe, M., Nussaume, O., & Andreassian, B. (1988). Renal artery emboli: the role of surgical treatment. Annals of vascular surgery, 2(2), 161-168.
View at Publisher | View at Google Scholar - Silverberg, D., Menes, T., Rimon, U., Salomon, O., & Halak, M. (2016). Acute renal artery occlusion: Presentation, treatment, and outcome. Journal of vascular surgery, 64(4), 1026-1032.
View at Publisher | View at Google Scholar - Siablis, D., Liatsikos, E. N., Goumenos, D., Karnabatidis, D., Voudoukis, T., Barbalias, G., & Vlahogiannis, J. (2005). Percutaneous rheolytic thrombectomy for treatment of acute renal-artery thrombosis. Journal of endourology, 19(1), 68-71.
View at Publisher | View at Google Scholar - Greenberg, J. M., Steiner, M. A., & Marshall, J. J. (2002). Acute renal artery thrombosis treated by percutaneous rheolytic thrombectomy. Catheterization and cardiovascular interventions, 56(1), 66-68.
View at Publisher | View at Google Scholar - Pellerin, O., Garçon, P., Beyssen, B., Raynaud, A., Rossignol, P., Jacquot, C., ... & Sapoval, M. (2009). Spontaneous renal artery dissection: long-term outcomes after endovascular stent placement. Journal of Vascular and Interventional Radiology, 20(8), 1024-1030.
View at Publisher | View at Google Scholar - Karakurt, A. (2018). New thrombolytic infusion application of dissolving renal artery embolic thrombosis: low-dose slow-infusion thrombolytic therapy. Case Reports in Nephrology, 2018.
View at Publisher | View at Google Scholar - Chondros, K., Karpathakis, N., Tsetis, D., Sofras, F., & Mamoulakis, C. (2014). Systemic thrombolysis with the use of tenecteplase for segmental acute renal in-farction potentially associated with multiple thrombophilic gene polymorphisms. Hippokratia, 18(1), 67.
View at Publisher | View at Google Scholar - Salam, T. A., Lumsden, A. B., & Martin, L. G. (1993). Local infusion of fibrinolytic agents for acute renal artery thromboembolism: report of ten cases. Annals of vascular surgery, 7(1), 21-26.
View at Publisher | View at Google Scholar - Heidemann, F., Kölbel, T., Debus, E. S., Diener, H., Carpenter, S. W., Rohlffs, F., & Tsilimparis, N. (2018). Renal function salvage after delayed endovascular revascularization of acute renal artery occlusion in patients with fenestrated-branched endovascular aneurysm repair or visceral debranching. Journal of Endovascular Therapy, 25(4), 466-473.
View at Publisher | View at Google Scholar - Steckel, A., Johnston, J., Fraley, D. S., Bruns, F. J., Segel, D. P., & Adler, S. (1984). The use of streptokinase to treat renal artery thromboembolism. American Journal of Kidney Diseases, 4(2), 166-170.
View at Publisher | View at Google Scholar - Rouvière, O., Berger, P., Béziat, C., Garnier, J. L., Lefrançois, N., Martin, X., & Lyonnet, D. (2002). Acute thrombosis of renal transplant artery: graft salvage by means of intra-arterial fibrinolysis. Transplantation, 73(3), 403-409.
View at Publisher | View at Google Scholar - Cheng, B. C., Ko, S. F., Chuang, F. R., Lee, C. H., Chen, J. B., & Hsu, K. T. (2003). Successful management of acute renal artery thromboembolism by intra-arterial thrombolytic therapy with recombinant tissue plasminogen activator. Renal failure, 25(4), 665-670.
View at Publisher | View at Google Scholar - Nakayama, T., Okaneya, T., Kinebuchi, Y., Murata, Y., & Iizuka, K. (2006). Thrombolytic therapy for traumatic unilateral renal artery thrombosis. International journal of urology, 13(2), 168-170.
View at Publisher | View at Google Scholar - Siezenga, M. A., van Overhagen, H., & van Buren, M. (2005). Acute occlusion of the renal artery treated by means of rheolytic thrombectomy. Nederlands Tijdschrift voor Geneeskunde, 149(43), 2413-2417.
View at Publisher | View at Google Scholar - Moyer, J. D., Rao, C. N., Widrich, W. C., & Olsson, C. A. (1973). Conservative management of renal artery embolus. The Journal of Urology, 109(2), 138-143.
View at Publisher | View at Google Scholar - Ayach, T., & Kazory, A. (2013). Bilateral renal infarction: an uncommon presentation of fibromuscular dysplasia. Clinical kidney journal, 6(6), 646-649.
View at Publisher | View at Google Scholar - Hokanson, J. E., & Austin, M. A. (1996). Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. Journal of cardiovascular risk, 3(2), 213-219.
View at Publisher | View at Google Scholar - Brunzell, J. D. (2007). Hypertriglyceridemia. New England Journal of Medicine, 357(10), 1009-1017.
View at Publisher | View at Google Scholar