case report | DOI: https://doi.org/10.58489/2836-5917/013
Case report of acute pericarditis-associated Atrial Fibrillation and thromboembolism MI with Non-Obstructive Coronary Arteries (MINOCA)
1American University of the Caribbean, School of Medicine, University Drive at, Jordan Dr, Cupecoy, Sint Maarten
2Cardiology Department, Stepping Hill Hospital, Stockport, UK
3Division of Cardiovascular Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Core Technology Facility, 46 Grafton Street, Manchester M13 9NT, UK
*Corresponding Author: Nadim Malik
Citation: Bradley Mueller, Joanna Highstein, Matthew Saxton, Cathy M Holt & Nadim Malik. (2023), Case report of acute pericarditis-associated Atrial Fibrillation and thromboembolism MI with Non-Obstructive Coronary Arteries (MINOCA). 2(2). DOI: 10.58489/2836-5917/013
Copyright: © 2023 Nadim Malik, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 29 June 2023 | Accepted: 18 July 2023 | Published: 04 August 2023
Keywords: Acute pericarditis, Atrial fibrillation (AF), thromboembolism, Type 2 MI, MINOCA.
Abstract
Myocardial Infarction (MI) with non-obstructive coronary artery disease (MINOCA), defined as MI without coronary artery stenosis ≥50% on invasive angiography, makes up around 6-14% of all MI presentations with one-year all-cause mortality of 4.7%. MINOCA encompasses a heterogeneous group of conditions. Although patients with MINOCA are more likely to have associated hypercoagulable states, they have a better prognosis than patients with obstructive coronary artery disease (CAD) MI. However, 1 in 4 patients with MINOCA may experience another cardiac event in subsequent years, with women having a worse outcome than men. Treatment for MINOCA should therefore be tailored towards the precipitating aetiology as well as secondary prevention of MI for longer-term benefits. We present a case of first episode of acute pericarditis associated with new persistent atrial fibrillation (AF) leading to thromboembolic MINOCA, and highlight the management decisions to individualise treatments for the various issues specific to this case.
Key learning
- Acute pericarditis-associated AF, described as uncommon and usually short-lived, can become persistent
- AF related thromboembolism risk is unchanged irrespective of aetiology
Use of multi-modality investigations to diagnose Type 2 MI/MINOCA help to personalise treatments including secondary prevention of CAD
Introduction
Myocardial Infarction (MI) with non-obstructive coronary artery disease (MINOCA), defined as MI without coronary artery stenosis ≥50% on invasive angiography (1, 2), makes up around 6-14% of all MI presentations with one-year all-cause mortality of 4.7% (3). MINOCA encompasses a heterogeneous group of conditions. Although patients with MINOCA are more likely to have associated hypercoagulable states, they have a better prognosis than patients with obstructive coronary artery disease (CAD) MI [4]. However, 1 in 4 patients with MINOCA may experience another cardiac event in subsequent years, with women having a worse outcome than men [4]. Treatment for MINOCA should therefore be tailored towards the precipitating aetiology as well as secondary prevention of MI for longer-term benefits [4].
We present a case of first episode of acute pericarditis associated with new persistent atrial fibrillation (AF) leading to thromboembolic MINOCA, and highlight the management decisions to individualise treatments for the various conditions specific to this case.
Key learning
- Acute pericarditis-associated AF, described as uncommon and usually short-lived, can become persistent
- AF related thromboembolism risk is unchanged irrespective of aetiology
Use of multi-modality investigations to diagnose Type 2 MI/MINOCA help to personalise treatments including secondary prevention of CAD
Case Report
A 69-year-old male, retired software engineer, presented to Emergency Department with history of palpitations (described as very fast heart beat in chest) and sweating but no nausea or shortness of breath for a few days, followed by a two-day history of central, severe (9/10 intensity) chest pains. The initial episode was sudden onset, at rest, felt like ‘exploding chest’, but then became constant, worse on lying flat and deep inspiration, eased by leaning forward. Past history included hypertension, benign prostatic hypertrophy (BPH), gout and gastro-oesophageal reflux disease. A lifelong non-smoker, he averaged 10 units of alcohol/week with no family history of premature CAD or sudden cardiac death. Medication on admission were omeprazole, losartan and allopurinol.
Initial examination confirmed BMI 28.5 (weight 83.6kg), irregularly-irregular pulse at 114/minutes (tachycardia), BP 166/95mmHg (hypertensive), Temperature 390C (pyrexia), respiratory rate 18/minute and oxygen saturation 99% on room air. He was euvolaemic, heart sounds were normal with no audible pericardial or pleural rubs, chest was clear and abdomen was soft with bowel sounds present. Given the severity of the pain, he required opiate analgesia and sublingual GTN. The chest pain responded partially to sublingual GTN, with additional benefit from regular paracetamol/codine over the next 2 days.
Initial differential diagnosis included acute aortic dissection, acute coronary syndrome and acute pericarditis/myopericarditis with new atrial fibrillation. Investigations confirmed an elevated C-reactive protein (CRP) with normal white cell count, but both increasing in subsequent days (Table. 1), normal haemoglobin, platelet count, renal function, thyroid function and magnesium levels. High-sensitivity troponin I was raised. Urinalysis (MSU) and Chest radiography (CXR) were normal. Blood cultures, full viral screen and auto-antibody screen were all negative.
Table 1: Results
| Day of Admission | Day 1 | Day 2 | Day 3 | Day 5 | Day 10 |
| Temperature (Celsius) | 39 | 37.9 | 37.6 | 37.4 | 37.3 |
| C-reactive protein (mg/l) (Normal <14> | 57.4 | 83.6 | 255 | 80 | 19 |
| White Cell Count (x 109L) (Normal 4.5-11) | 9.9 | 12.7 | 12.6 | 10.6 | 10.5 |
| Hs-Troponin (ng/mL) (Normal <50> | 676 |
|
|
|
|
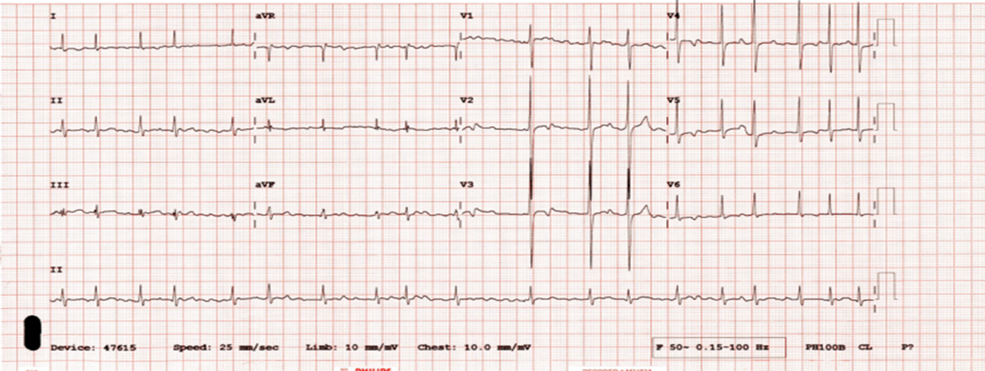
Electrocardiogram/ECG (Figure 1) confirmed AF with ventricular rate of 120bpm and non-specific T-wave changes
Urgent CT aortogram excluded acute aortic syndrome/dissection, but demonstrated diffuse coronary artery calcification with atheroma and CT chest/abdomen identified ‘multifocal sites of non-occlusive thrombus’ in the superior mesenteric artery (SMA) without evidence of bowel ischaemia, and no evidence of any other source of infection in chest/abdomen.
Working diagnosis included acute pericarditis with new AF, hypertension and type 2 non-ST elevation myocardial infarction (NSTEMI). Treatment given included low-molecular weight heparin, dual antiplatelet therapy (aspirin, clopidogrel), bisoprolol, atrovastatin, losartan, omeprazole and colchicine. AF rate control was not achieved with maximum dose of bisoprolol, therefore amiodarone was started. Risk for thromboembolism was moderate (CHA2DS2VASC = 4) and risk for bleeding (HASBLED =4) was also moderate. Vascular surgery review of non-occlusive SMA thrombus advised formal anti-coagulation only.
Table 2: Tests and results of cardiac investigations are summarized
Test | Day performed | Key findings | Summary Outcome | Treatment change |
TTE | Day-2 after admission |
| Dilated LA | none |
Invasive coronary angiogram | Day-12 after admission |
| NSTEMI with Non-obstructive CAD | Secondary prevention for type 2 MI |
CMR with perfusion study | 2-months after discharge |
| Dilated LA |
|
Ambulatory 72 hours ECG | 3-months after discharge |
|
| duration of NOAC |
Final diagnosis and treatment
Given the classical history of two different but co-existing types of chest pains (‘anginal’ and ‘pericarditic’) at presentation, the hypothesized unifying diagnosis was of acute pericarditis, possibly viral etiology, with new persistent AF and multiple thromboembolic events – multi-focal clots in SMA and type 2 MI. With angiographic confirmation of non-obstructive coronary arteries only, final diagnosis was refined to MINOCA.
Final treatment included oral anticoagulation (edoxaban 60mg), amiodarone for rate/rhythm control with aggressive secondary prevention of CAD (single anti-platelet agent, beta blockers, angiotensin receptor blockade and high dose statin) as per initial management above. For ongoing pains of pericarditis, colchicine was advised for a minimum of three months. After 6-weeks on amiodarone the liver function tests were reported to be mildly abnormal, and the AF was noted to have chemically cardioverted to sinus rhythm. Amiodarone was therefore ceased. He successfully completed post-MI cardiac rehabilitation programme. Out-patient clinic follow up at 3 months after discharge confirmed he was asymptomatic, in persistent sinus rhythm and was therefore discharged from further cardiology care.
Discussion
This case presents 2 key learning points. Frist, it highlights the shared common pathophysiology of the various cardiac conditions/diagnosis identified within one patient: acute pericarditis, new atrial fibrillation with thromboembolic manifestations including MI. Second, this case demonstrates the conundrum and confusion over the use of common but overlapping current used definitions/classifications of MI whilst also identifying their utility in developing personalized management of the specific pathophysiology contributing towards each underlying diagnosis.
The presumed initial etiology in this case, acute pericarditis, typically presents with two or more of the following features: pleuritic/pericarditis-type chest pain, a friction rub and/or signs of pericardial effusion not uncommonly with a history of preceding infection, often viral, such as fever, cough/sore throat. Additionally, signs of a preceding infection including elevated C-reactive protein (CRP) and an abnormal white cell count may indicate plausible acute pericarditis, although their absence would not necessarily exclude such diagnosis [5]. Our case demonstrated such features at presentation. Suggestive ECG changes can include PR segment depression or saddle-shaped diffuse ST elevations but in our patient, these were absent [5]. Kyoto et al reported that men are more likely to be affected with acute pericarditis [6], as in this case. Management of uncomplicated acute pericarditis, which is a self-limiting condition, is primarily pain relief with high doses of non-steroidal anti-inflammatory drugs along with use of colchicine to reduce risks of recurrences [7, 8].
Pericarditis related atrial fibrillation (AF)
Not all cases of acute pericarditis are however uncomplicated, with some cases developing pericardial effusions with/without associated cardiac dysrhythmias. This patient developed AF without any effusion. The association of AF with acute pericarditis is described as being uncommon since early studies [9], and this trend is noted even in more recent studies [10, 11]. Irrespective, AF is recognized as the most frequent arrhythmia associated with acute pericarditis [11]. Spodick reported that pericarditis-related AF was more likely to occur in patients with pre-existing underlying heart disease [12]. In 2015, Imazio et al, however reported pericarditis-associated AF to more likely occur in patients with pre-existing hypertension (HT) or dilated left atria, with the older populations also more likely to develop pericardial effusions [11]. This patient had both HT and dilated left atrium. Likewise, cases of pericarditis-associated AF were more likely to present with a fever and elevated N-terminal pro-brain natriuretic peptide (BNP) result, compared to those who did not develop AF [10, 11]. Imazio and colleagues have previously also reported that the AF occurs usually within 24 hours of onset of acute pericarditis, and is usually also short lived (i.e. < 24 xss=removed>[11]. In this patient, the AF was persistent but eventually responded to chemical cardioversion. We hypothesize that the pericarditis-associated AF contributed to other clinical features of this case i.e. multiorgan thromboembolism (bowel and heart), which then required investigations within specific individualized pathways, eventually leading to the final diagnosis of type 2 MI/MINOCA.
Type 2 MI or MINOCA or both?
The basic established criteria for diagnosis of acute MI include presence of symptoms of ischemia, ST/T segment changes on an electrocardiograph (ECG) and a rise in cardiac specific enzymes (confirmatory of myocardial cell death). In 2007, a global task force redefined MI with the introduction of five subclasses of MI [13] and with further refinements ‘The fourth universal definition of MI’ was adopted for use from 2018 onwards with the aim to help direct specific treatments of the various often co-existing/common patho-physiological mechanisms leading to the MI [14]. Accordingly, the present case would be classed and treated as Type 2 MI. Typically, Type 2 MI occur due to mismatch between supply and demand (of oxygen or blood) to the heart muscle causing ischemic injury. Acute stressors leading to type 2 MI would include generalized causes such as acute gastrointestinal blood loss, severe anemia, respiratory failure, sepsis, hypotension/shock, acute aortic dissection, embolism (of thrombi, calcium or vegetation from atria, ventricles or valves) and any sustained tachy- or brady- arrhythmias. In addition, most cases of type 2 MI exhibit underlying established coronary artery disease (CAD), identified by angiography as obstructive (³ 50%) or non-obstructive (£50%), but is also reported in the absence of CAD (i.e. normal coronary arteries) which helps explain the substantial variability reported in the severity of the stressor and/or extent of ischemic myocardial injury (MI) noted in such cases.
However, once apparent, systemic causes for the type 2 MI (mentioned above) have been excluded, the ischemic injury (MI) can then be re-evaluated under another commonly used and overlapping terminology to direct more individualized/ personalized and targeted treatment for the MI. The specific terminology used to describe the MI with underlying non-obstructive CAD (i.e. angiographic CAD of £ 50% in all major epicardial arteries) is MINOCA [3]. MINOCA presents with clinical features of ischemia (anginal chest pain with response to GTN), associated with raised cardiac marker/enzymes (Troponin) with/without ECG changes, with confirmation of the non-obstructive anatomy only on coronary angiography. A diagnosis of MINOCA therefore warrants involvement of multiple cardiac sub-specialists, for multimodality imaging (e.g. CMR) and arrhythmias assessment in addition to coronary interventional specialists to help identify the precise underlying cause/extent of MI injury. Such risk stratification then also directs formulation of a personalized treatment plan.
For example, in MINOCA with confirmed non-obstructive CAD only the use of standard secondary prevention therapy is known to be prognostically beneficial [15]. However, in MINOCA, assumed to be due to coronary embolism, the treatment of the hypercoagulable state, with formal anticoagulant (e.g. NOAC) would also be appropriate along with secondary preventive care [16]. In summary, whilst the presence of any non-obstructive (i.e. non-culprit) CAD dictates use of accepted secondary prevention (with its well-defined advantages), additional therapies targeting the specific reason for supply-demand mismatch causing the ischemic injury (MI) should not be overlooked.
The present case was therefore managed as MINOCA with standard secondary preventive treatment as well as NOAC for the AF-related thromboembolism. However, what remains unknown in such cases is the necessary duration for NOAC therapy, especially as the incidence of recurrent AF in such cases is unknown. The patient in this case report, with history of pre-existing HT and dilated left atria (CHA2DS2VASC = 4), is likely to benefit from longer-term anticoagulation for protection from further/future embolic events. Likewise, it would not be unreasonable to consider longer-term rhythm monitoring (e.g. Internal Loop recorder implantation) of cases where the risk of recurrent embolism is lower to help determine the ideal duration of anticoagulation.
In summary, this case highlights the conundrum in implementing guideline-directed optimal therapies (GDOT) in real world patients with multiple/co-existing patho-physiologies and the usefulness of the newer classifications for MI to formulate personalize treatments.
References
- Singh, T., Chapman, A. R., Dweck, M. R., Mills, N. L., & Newby, D. E. (2021). MINOCA: a heterogenous group of conditions associated with myocardial damage. Heart, 107(18), 1458-1464.
View at Publisher | View at Google Scholar - Agewall, S., Beltrame, J. F., Reynolds, H. R., Niessner, A., Rosano, G., Caforio, A. L., ... & WG on Cardiovascular Pharmacotherapy. (2017). ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. European heart journal, 38(3), 143-153.
View at Publisher | View at Google Scholar - Nordenskjöld, A. M., Baron, T., Eggers, K. M., Jernberg, T., & Lindahl, B. (2018). Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. International journal of cardiology, 261, 18-23.
View at Publisher | View at Google Scholar - Safdar, B., Spatz, E. S., Dreyer, R. P., Beltrame, J. F., Lichtman, J. H., Spertus, J. A., ... & D'Onofrio, G. (2018). Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. Journal of the American Heart Association, 7(13), e009174.
View at Publisher | View at Google Scholar - Imazio, M., Brucato, A., Maestroni, S., Cumetti, D., Dominelli, A., Natale, G., & Trinchero, R. (2011). Prevalence of C-reactive protein elevation and time course of normalization in acute pericarditis: implications for the diagnosis, therapy, and prognosis of pericarditis. Circulation, 123(10), 1092-1097.
View at Publisher | View at Google Scholar - Kytö, V., Sipilä, J., & Rautava, P. (2014). Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation, 130(18), 1601-1606.
View at Publisher | View at Google Scholar - Lilly L. S. (2013). Treatment of acute and recurrent idiopathic pericarditis. Circulation, 127(16), 1723–1726.
View at Publisher | View at Google Scholar - Adler, Y., Charron, P., Imazio, M., Badano, L., Barón-Esquivias, G., Bogaert, J., Brucato, A., Gueret, P., Klingel, K., Lionis, C., Maisch, B., Mayosi, B., Pavie, A., Ristic, A. D., Sabaté Tenas, M., Seferovic, P., Swedberg, K., Tomkowski, W., & ESC Scientific Document Group (2015). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). European heart journal, 36(42), 2921–2964.
View at Publisher | View at Google Scholar - Spodick, D. H. (1976). Arrhythmias during acute pericarditis: a prospective study of 100 consecutive cases. Jama, 235(1), 39-41.
View at Publisher | View at Google Scholar - Mayosi B. M. (2015). Pericarditis-associated atrial fibrillation. Heart (British Cardiac Society), 101(18), 1439–1440.
View at Publisher | View at Google Scholar - Imazio, M., Lazaros, G., Picardi, E., Vasileiou, P., Orlando, F., Carraro, M., ... & Gaita, F. (2015). Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart, 101(18), 1463-1467.
View at Publisher | View at Google Scholar - Spodick, D. H. (1998). Significant arrhythmias during pericarditis are due to concomitant heart disease. Journal of the American College of Cardiology, 32(2), 551-552.
View at Publisher | View at Google Scholar - Thygesen, K., Alpert, J. S., White, H. D., & Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007). Universal definition of myocardial infarction. European heart journal, 28(20), 2525–2538.
View at Publisher | View at Google Scholar - Thygesen, K., Alpert, J. S., Jaffe, A. S., Chaitman, B. R., Bax, J. J., Morrow, D. A., White, H. D., Document Group, E. S., Thygesen, K., Alpert, J. S., Jaffe, A. S., Chaitman, B. R., Bax, J. J., Morrow, D. A., White, H. D., Mickley, H., Crea, F., Katus, H. A., Pinto, F. J., . . . Corbett, S. (2019). Fourth universal definition of myocardial infarction (2018). European Heart Journal, 40(3), 237-269.
View at Publisher | View at Google Scholar - Nordenskjöld, A. M., Agewall, S., Atar, D., Baron, T., Beltrame, J., Bergström, O., Erlinge, D., Gale, C. P., López-Pais, J., Jernberg, T., Johansson, P., Ravn-Fisher, A., Reynolds, H. R., Somaratne, J. B., Tornvall, P., & Lindahl, B. (2021). Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. American heart journal, 231, 96–104.
View at Publisher | View at Google Scholar - Niccoli, G., & Camici, P. G. (2020). Myocardial infarction with non-obstructive coronary arteries: what is the prognosis?. European heart journal supplements, 22(Supplement_E), E40-E45.
View at Publisher | View at Google Scholar