Research Article | DOI: https://doi.org/10.58489/2836-8959/009
Another patient with symptomatic uremia treated with intestinal dialysis: An educational article
- Aamir Jalal Al-Mosawi 1
1 Advisor doctor and expert trainer The National Training and Development Center and Baghdad Medical City
*Corresponding Author: Aamir Jalal Al-Mosawi
Citation: Aamir Jalal Al-Mosawi, 2023 Another patient with symptomatic uremia treated with intestinal dialysis: An educational article. Journal of Clinical Sciences and Clinical Research.2(1). DOI: 10.58489/2836-8959/009
Copyright: © 2023 Aamir Jalal Al-Mosawi, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 03 March 2023 | Accepted: 10 March 2023 | Published: 14 March 2023
Keywords: Symptomatic uremia, intestinal dialysis, educational article.
Abstract
Background: Peritoneal dialysis actually acts by shifting the urinary excretion of urea to the peritoneal excretion with use of intraperitoneal dialysis fluids. Intestinal dialysis acts by shifting the urinary excretion of urea to intestinal excretion by modifying the enterhepatic urea cycle and increasing the amount of nitrogen eliminated as fecal waste. During intestinal dialysis, the patient consumes a relatively large amount of soluble fiber “acacia gum” which is digested by colonic flora, and cause a shift of urinary excretion. We have previously reported in several publications our pioneering and extensive experience with intestinal dialysis.
Patients and methods: The case of 57 years old man with symptomatic uremia who refused hemodialysis treatment and treated with intestinal dialysis is described.
Results: One week treatment with intestinal dialysis was associated with marked symptomatic and laboratory improvements. Blood urea was lowered from 162.4 mg/dL to 47 mg /dL, and serum creatine was lowered from 4.5 mg/dL to 1.1 mg /dL.
Conclusion: Intestinal dialysis components include a urea lowering agent, powdered acacia gum, the dietary therapies of chronic renal failure, and the pharmacologic therapies of chronic renal failure which are also used during management with chronic hemodialysis and peritoneal dialysis. This paper reports another case showing that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure.
Introduction
Chronic renal failure is associated with development of the uremic syndrome which is the signs and symptoms resulting from the retention of nitrogenous waste products, imbalance in the body content and distribution of water and electrolytes, and an inadequate production of renal hormones. The most prominent features of the uremic syndrome (nausea, anorexia, vomiting and fatigue) result from nitrogen retention with an increase in plasma concentration of the products of protein metabolism especially urea.
Ingested proteins are degraded into amino acids and their metabolism by the liver yield nitrogen, mainly in the form of urea.
Several products of protein metabolism have been identified, with urea being quantitively the most important. Urea represents 80% or more of the total nitrogen excreted into the urine in patients with chronic renal failure maintained on diets containing 40 g or more of protein. Approximately 25-40 % of the manufactured urea are recycled through the gastrointestinal tract and excreted fecally. This extra-renal excretion of urea remains constant at less than 3 g daily even in uremia despite the marked increases in blood urea.
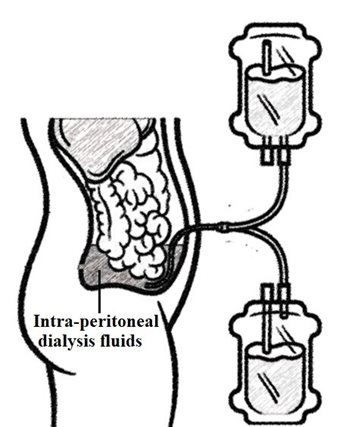
Peritoneal dialysis actually acts by shifting the urinary excretion of urea to the peritoneal excretion with use of intraperitoneal dialysis fluids (Figure-1).
Intestinal dialysis acts by shifting the urinary excretion of urea to intestinal excretion by modifying the enterhepatic urea cycle and increasing the amount of nitrogen eliminated as fecal waste. During intestinal dialysis, the patient consumes a relatively large amount of soluble fiber “acacia gum” which is digested by colonic flora, and cause a shift of urinary excretion [1-15].
Patients and methods
A 57-year diabetic male was experiencing progressive symptomatic uremia with nausea, vomiting, fatigue, and anemia over the previous weeks. His diabetes was controlled with oral anti-diabetic drug and was also receiving oral isosorbide dinitrate 10 mg once daily and low dose aspirin because of having previously an evidence of ischemic heart disease. During the course of his illness he was experiencing reduction of the urine output associated with peripheral edema. However, urine output improved with treatment with frusemide 125 mg once daily and bumetanide 1 mg daily.
On the 24 of December, 2022, blood urea elevated at was 162.4 mg/dL, serum creatine was 4.5 mg/dL, and he was anemic with hemoglobin at 8 g/dL (Normal ranges: 11.5-16.5 g/dL). Urinalysis didn’t show important findings. He was seen by more than two doctors and all referred him for hemodialysis treatment which he and his family consistently rejected.
Therefore, he was referred to us on the 31st of December, 2022 because of worsening of the uremic symptoms and refusal to dialysis therapy.
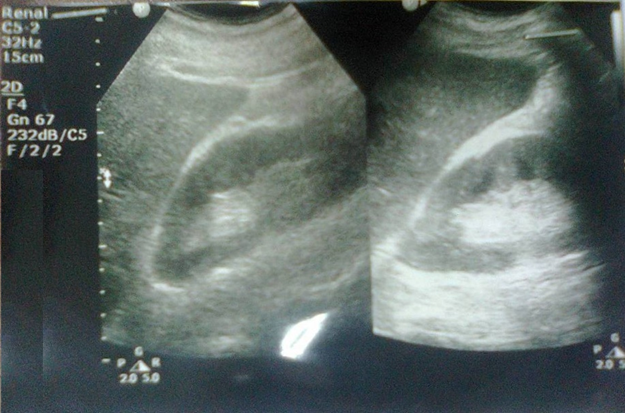
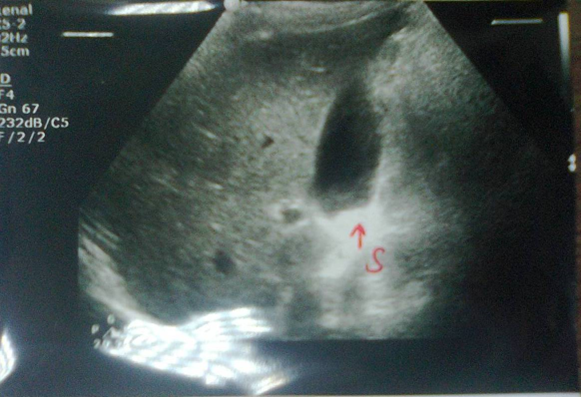
Renal ultrasound (Figure-2A) showed reduction in renal size (RK: 9.5 x 4.5 cm, cortex 17 cm, LK: 9.0 x 4.5, cortex 17 cm) [Adults male normal renal size is 10 x 9-14x13 cm]. Both kidneys showed hyper-echoic texture with intact cortical thickness, but with indistinct cortico-medullary differentiation.
Abdominal ultrasound (Figure-2B) showed small gall bladder stones (Average 2 mm) collected within the dependent part of the gall bladder. The gall bladder was thin and there was no evidence of cholecystitis.
The patient was treated with intestinal dialysis (Acacia gum supplementation plus very low protein diet) and pharmacological managements were prescribed according to the latest published guidelines [12, 15, 16, 17] and included oral calcium carbonate. Initially, the anemia was treated with intramuscular iron dextran 100 mg daily.
Results
The patient was seen again on the 7th of January, 2023 (Figure-3), treatment was associated with marked symptomatic and laboratory improvements. Blood urea was 47 mg /dL, serum creatine was 1.1 mg /dL, and he was anemic with hemoglobin at 8 g/dL (Normal ranges: 11.5-16.5 g/dL). Serum calcium was 7.7 mg/dL (Normal ranges: 8-10.5 mg/dL). Therefore, oral alphacalcidol was added in a dose of 1 microgram daily.
Discussion
Management of non-terminal chronic renal failure is largely directed at compensating the diminished renal functions conservatively through dietary and pharmacologic measures which include protein and phosphorus restriction, calorie and water soluble vitamins supplementation, phosphate binders, and correction of other abnormalities that may be associated with chronic renal failure such as fluid and salt retention, hypocalcaemia, hyperkalemia and hypertension. The dietary interventions in chronic renal failure are directed at diminishing the accumulation of the products of nitrogen metabolism which are responsible for many of the symptoms and disturbances of uremia, and thus preventing the development of the uremic symptoms complications. The most essential strategy for diminishing the accumulation of the products of nitrogen metabolism in chronic renal failure is dietary protein restriction and this can be achieved safely with avoiding consequences of protein deficiency through the provision of low quantity of high quality protein diet.
High biologic value proteins reduce the accumulation of nitrogenous waste products and acid load for renal excretion and also help in maintaining a positive nitrogen balance. Egg protein has the highest biologic value (1 large egg contains 8 g protein). However, it’s very difficult and not really practical to limit the intake of proteins only to the high biologic value egg protein, and preferably 50% of the protein intake should be provided as egg, and 50% provided with the intake of vegetables, rice, and bread. Thus, avoiding restriction of caloric intake which may lead to negative nitrogen balance, muscle wasting and growth failure in case of children.
Protein restriction decreases endogenous protein catabolism and the accumulation of nitrogenous waste products, also reduces the phosphorus load and thus may prevent the onset of secondary hyperparathyroidism and renal oseodysrtophy. Protein restriction also reduces the intake of potential hydrogen ion, because every 10 g of protein provides 7 meq of hydrogen ion in the form of sulfated amino-acids .Therefore, protein restriction helps to ameliorate acidosis.
The second necessary strategy for diminishing the accumulation of the products of nitrogen metabolism is the adequate intake of carbohydrate and fats for their protein sparing effect which decreases endogenous protein catabolism and the production of urea. Fats are less effective than carbohydrates in minimizing protein catabolism, but they are given to provide essential fatty acids and to enhance the palatability of the diet. Dietary restrictions of sodium and potassium depend on the presence of oliguria, edema, hypertension, and hyperkalemia.
Pharmacologic measures used to prevent or correct abnormalities which are expected to appear during the course of chronic renal failure including anemia, iron and vitamins deficiencies, hypocalcaemia, hyperphosphatemia, hypertension, acidosis, growth retardation, renal oseodysrtophy. With appropriate dietary and pharmacologic management, patients with non-terminal chronic renal failure can be maintained surprisingly well and the transition from non-terminal chronic renal failure to end-stage chronic renal failure represents a small decrement of renal function resulting in a large physiologic hurdle for the patient.
Dietary and pharmacologic measures are only successful in non-terminal chronic renal failure patients. The addition to these effective traditional measures, a dietary agent that enhances extra-renal fecal nitrogen excretion can possibly bridge this gap resulting from this small decrement of renal function and obviating the need for dialysis for some period of time [1-18].
As early as 1980, Hippocrates Yatzidis (Figure-4A) and his research group showed that dietary fiber (locust bean gum) supplementation of chronic renal failure patients on low-protein diet reduced serum urea and creatinine by 11-23% over 5 days of intake. Improvement of clinical symptoms was observed during this period and deterioration was observed when supplementation was stopped [6, 7].
In 1984, Rampton et al reported that six and 8 weeks of ispaghula and arabinogalactan reduced mean plasma urea level in uremic patients 19% and 11% respectively. However, the use of locust bean gum and ispaghula were not considered to have practical value for clinical use because of the development of side effects. The use of locust bean gum was associated with two to three soft voluminous stools daily that had an unusual bad smell, and more importantly, the low palatability was considered a serious disadvantage. Similarly patients ingested ispaghula found it unpalatable, felt unwell, or had diarrhea.
In 1996, Tetens et al showed in an experimental study on rats that dietary fiber (maize bran) supplementation caused a dose dependent significant increase in fecal nitrogen excretion in association with reduction in nitrogen retention. The shift in nitrogen excretion from urine to feces was explained largely by the degree of microbial fermentation in the large intestine caused by dietary fiber that have modifying role on the enterohepatic cycle of nitrogen.
In 1996, Donna Bliss from the United States (Figure-4B) and the supervisors of her PhD dissertation reported that acacia gum lowered urea level in patients with asymptomatic chronic renal failure on low protein diet by increasing fecal nitrogen excretion. In contrast to locust bean gum and ispaghula, patients found acacia gum palatable and its clinical use was considered applicable [6, 7, 8, 9].
Intestinal dialysis components include a urea lowering agent, powdered acacia gum, the dietary therapies of chronic renal failure, and the pharmacologic therapies of chronic renal failure which are also used during management with chronic hemodialysis and peritoneal dialysis [10, 11, 12, 13, 14].
The combined use of intestinal dialysis with intermittent peritoneal dialysis was the first reported use of intestinal dialysis in symptomatic uremia. In this paper, the use of intestinal dialysis plus intermittent peritoneal dialysis to treat a seven year old boy with an extreme form of end-stage renal disease (anuric with no renal function) was described [1].
In 2004, the achievement of 1-year dialysis freedom with intestinal dialysis in patients who had symptomatic uremia that required at least one session of intermittent peritoneal dialysis despite low protein diet and other conservative measures of symptomatic uremia was reported. In this paper, patients treated with intestinal dialysis reported improved wellbeing. Neither became acidotic or uremic, and didn’t require dialysis with maintaining serum creatinine and urea levels not previously achieved without dialysis [2].Thereafter, long-term dialysis freedom has been reported in patients with symptomatic uremia treated with intestinal dialysis [3,4,10].
Powdered acacia gum (Figure-5A) is a complex polysaccharide obtained mostly from the dried gummy material (Figure-5B) of the stem and branches of acacia trees, Senegal family leguminosae (Figure-5C).It is recognized as safe by the FDA. It’s widely used in the production of foods such as puddings, candy, and beverages. It has demulcent properties and therefore, it is often added to medicines.
Conclusion
Intestinal dialysis components include a urea lowering agent, powdered acacia gum, the dietary therapies of chronic renal failure, and the pharmacologic therapies of chronic renal failure which are also used during management with chronic hemodialysis and peritoneal dialysis. This paper reports an other case showing that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure.
Acknowledgement
The patient kindly accepted publishing his photo to enhance the documentation of the paper.
The author has the copyright of all the sketches in this paper.
Conflict of interest: None.
References
- Al-Mosawi AJ. (2002) The challenge of chronic renal failure in the developing world: possible use of acacia gum. Pediatr Nephrol May; 17(5):390-1. Doi: 10.1007/s00467-001-0755-4. PMID: 12042902.
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2004) Acacia gum supplementation of a low-protein diet in children with end-stage renal disease. Pediatr Nephrol. Oct; 19(10):1156-9. Doi: 10.1007/s00467-004-1562-5.PMID: 15293039.
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2007) The use of acacia gum in end stage renal failure. J Trop Pediatr Oct; 53(5):362-5. Doi: 10.1093/tropej/fmm033. PMID: 17517814.
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2009) Six-year dialysis freedom in end-stage renal disease. Clin Exp Nephrol Oct; 13(5):494-500. Doi: 10.1007/s10157-009-0181-7. PMID: 19479191.
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2006) Continuous renal replacement in the developing world: Is there any alternative. Therapy (Clinical practice) [p-ISSN: 2044-9038, e-ISSN: 2044-9046] Mar:3(2): 265-272. Doi: 10.2217/ 14750708.3.2.265
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2011) Intestinal dialysis: A new therapy for chronic renal failure. 1st ed., Saarbrücken; LAP Lambert Academic Publishing: (ISBN: 9783847304470).
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2013) Advances in peritoneal dialysis. In, Ed, Peritoneal Dialysis: Types, Procedures and Risks. Nova Science Publisher, New York, 1st Edition, January, (ISBN-10:1622578961, ISBN-13:978-1622578 962). Doi:10.13140/rg.2.1.4315.1120
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2013) A new dietary therapy for chronic renal failure .1st ed., Saarbrücken; LAP Lambert Academic Publishing: (ISBN: 978-3-659-51436-4).
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2017) Advances of peritoneal dialysis in the developing world: Combined intermittent peritoneal dialysis and intestinal dialysis (Chapter 3). In Marcia Bell, Ed, Peritoneal Dialysis: Practices, Complications and Outcomes. Nova Science Publisher, New York, (ISBN: 978-1-53610-515-5). Doi:10.13140/rg.2. 4315.1120
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2019) Dietary dialysis with acacia gum: Intestinal dialysis technology. Advancements in Journal of Urology and Nephrology (ISSN 2689-8616) Nov 18; 2(1): 1-8. Doi:10.33140/AJUN.0005
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) A New Dietary Therapy for Chronic Renal Failure: Intestinal Dialysis Technology. Journal of medical and surgical urology Jan; 1(1): 8-16. Doi: 10.5281/ zenodo.3878418
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) Intestinal (Dietary) Dialysis: A Practical Nutritional Guide. Journal of Urology and research (ISSN: 2379-951X) Aug; 7 (1): 1118 [1-3].
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) Intestinal dialysis in a uremic patient with diabetic nephropathy: A challenging case and a unique experience. Journal of Pharmaceutical Research and Development Aug; 20; 1 (1):1-4 Doi: 10.5281/zenodo.3977265
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) History of Medicine: The Emergence of Intestinal Dialysis. SunKrist Nephrology and Urology Journal August 22; 2 (1): 1-8. Doi: 10.46940/snuj.01002
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) Intestinal dietary dialysis: A practical treatment guide. 1st ed., Saarbrücken; LAP Lambert Academic Publishing: (ISBN: 978-3-659-87697-4).
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2020) Intestinale diätetische Dialyse: Ein praktischer Behandlungsleitfaden (German edition) Verlag Unser Vissen: (ISBN: 978-620-2-59519-3).
View at Publisher | View at Google Scholar - Al-Mosawi AJ. La dialyse (2020) intestinale alimentaire: Un guide pratique de traitement (French edition). Editions Notre Savoir: (ISBN-978-620-2-59521-6).
View at Publisher | View at Google Scholar - Al-Mosawi AJ. (2019) Scientific Publication Productivity and Research Activities of Iraqi Pediatricians in the Field of Pediatric Nephrology: A Bibliometric Analysis to Identify Pioneers. Advancements in Journal of Urology and Nephrology (ISSN 2689-8616) Nov 18; 1(1): 1-10. Doi.org/10.33140/AJUN.0006
View at Publisher | View at Google Scholar