Research Article | DOI: https://doi.org/10.58489/2836-2276/016
Aeromonas SPP In Sardinian Snail Farms
- Valentina Coroneo 1*
- Monica Bulla 2
- Alessia Noli 3
- Adriana Sanna 1
- Sara Salza 3
- Maria Paola Cogoni 2
- Department of Medical Sciences and Public Health, University of Cagliari, Cittadella Universitaria, S.P. 8, 09100 Cagliari, Italy.
- Istituto Zooprofilattico Sperimentale della Sardegna, Food and Water Microbiology Laboratory, Elmas (CA)
- Istituto Zooprofilattico Sperimentale della Sardegna, Microbiological food control and ispection Sassari (SS)
*Corresponding Author: Valentina Coroneo
Citation: Valentina Coroneo, Monica Bulla, Alessia Noli, Adriana Sanna, Sara Salza, Maria Paola Cogoni. (2023). Aeromonas SPP In Sardinian Snail Farms. Journal of Food and Nutrition. 2 (2); DOI: 10.58489/2836-2276/016
Copyright: © 2023 Valentina Coroneo, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 20 June 2023 | Accepted: 20 September 2023 | Published: 25 September 2023
Keywords: Aeromonas spp, virulence genes, snail farming, Helix aspersa Muller, watering.
Abstract
Snails, also known as Gastropod molluscs of the order Stilommatophoridae, belonging to the family Helicidae, are farmed in Sardinian agriculture. Nonetheless, this practice is currently causing numerous problems and critical issues. Setting up snail farming facilities demands high organisational standards to optimise production, as well as continuous in-depth technical training for dedicated personnel. The aim of this paper was to acquire knowledge on the epidemiology and ecology of Aeromonas spp. in snail farms and to assess the pathogenicity of the different strains, isolated through molecular characterisation of virulence genes. In order to do this, snails belonging to the genus and species Helix aspersa (N.22) and irrigation water (N.22) from various farms in Sardinia were sampled and microbiological culture investigations for Aeromonas spp. were carried out. The microorganisms isolated were subjected to culture and molecular investigations for the characterisation of virulence genes. From this study, it was possible to highlight the presence of 15 positive samples for Aeromonas spp. In addition, it was discovered that the origin of the contamination was often associated with unexpected factors, which were not under the control of those responsible for the plant. As a result, this study revealed the need for further preventive actions in two key aspects;
- improve the production in the surveillance of the farms;
- increase the assessment of associated risks caused by virulent strains that may compromise product quality and consumer safety.
Introduction
The genus Aeromonas consists of Gram-negative, rod-shaped, facultatively anaerobic, non-spore-forming, catalase- and oxidase-positive bacteria found in soil and aquatic environments. Most motile strains produce a single polar flagellum, while peritrichous or lateral flagella may form on solid media in some species. They grow at temperature ranges between 22 °C and 35 °C, and in some species, growth may also occur at 0-45 °C. There are more than 30 genetically diverse species with complex taxonomy. Current literature indicates that A. hydrophila, A. veronii bv sobria and A. caviae are responsible for the majority of human infections and clinical isolations [1]. Aeromonas hydrophila is a pathogenic microorganism, while the pathogenicity of Aeromonas caviae, and Aeromonas sobria is still being studied. Aeromonas sobria is a micro-organism that overlaps and complicates other infection states, and is capable of developing gastrointestinal pathology in humans [2]. The first case of Aeromonas spp. disease caused by snails dates back to 1994 and occurred in France [3]. In addition, several other cases of infections with septicaemia, meningitis, wound infections, peritonitis, hepatobiliary infections and necrotising fasciitis were reported [4]. Cytotoxic heat-labile enterotoxin (Act) is the main virulence factor of A. hydrophila and is responsible for haemolytic, cytotoxic and enterotoxic activities. Indeed, Haemolysis involves the formation of pores in the target cell membrane and the entry of water from the external medium, resulting in cell swelling and subsequent lysis [5]. The toxin interacts with erythrocyte membranes, inserts itself into the lipid bilayer and creates pores in the range of 1.14 to 2.8 nm. The cholesterol present on cell membranes acts as a receptor for Act, enabling its activation with subsequent oligomerisation and pore formation. The toxin's activity also includes tissue damage and elevated fluid secretion in intestinal epithelial cells, resulting from the induction of proinflammatory response in the target cells [6]. The Cytotonic heat-labile enterotoxin (Alt) and Cytotonic heat-stable enterotoxin (Ast), do not produce degeneration of the epithelium and act similarly to cholera toxin by raising levels of adenylated cAMP and prostaglandins in intestinal epithelial cells. This induces an efflux of chloride ions, which leads to osmotic leakage of water into the intestinal lumen causing diarrhoea. On the other hand, Cytotonic enterotoxins produced by Aeromonas spp. show variable reactivity to cholera antitoxin: the thermolabile enterotoxin alt (56°C for 10 min) does not show cross-reactivity with cholera antitoxin, whereas the thermostable enterotoxin Ast (56°C for 20 min) reacts with cholera antitoxin [7].
Aeromonas spp. possesses several haemolysins (AerA, Ahh1 and Asa1) that cause α- and β-haemolysis. Aerolysin is a β- haemolysin and is one of the best-characterised virulence factors. The most frequent haemolysin in Aeromonas strains is a thermolabile haemolysin (encoded by Ahh1) that exhibits increased haemolytic activity when it coexists with Aerolysin A. Most Aeromonas strains also produce a variety of extracellular enzymes, which can contribute to overall virulence, such as proteases, lipases, collagenases, nucleases, amylases, chitinases and elastases [8].
Aeromonas hydrophila, caviae and sobria are among the 'Motile Aeromonad species' frequently isolated from fresh water, treated or purified sewage, seawater, and water intended for human consumption. Isolation is also reported from seafood and meat products that may represent vehicles for the indirect transmission of infectious diseases in humans, particularly in immunocompromised individuals. The presence of Aeromonas spp. was detected in various environmental matrices, in particular, high isolation rates were found in snail samples and from different water sources, indicating these samples as likely reservoirs and sources of infection for humans. Low isolation rates were found in faeces from transient hosts such as poultry, cattle and humans [9]. Aeromonas spp. has been isolated in several plant species (cabbage, carrot, cucumber, aubergine, lettuce, onion, tomato, potato and spinach) with Aeromonas caviae as the most frequent species followed by A. hydrophila [10,11,12]. Indeed, the surface of vegetables can be contaminated by different microorganisms depending on the microbial population in their original environment, their condition, processing method, storage time and conditions [13]. The environment of a snail plant should ensure the absence of chemical, microbiological and physical contaminants that pose a risk to product quality due to contamination of meat. Furthermore, snails accumulate different types of contaminants in their tissues [14] and therefore for the construction of the facility, soil and irrigation water must be analyzed to check for the presence of any environmental contaminants that may affect the quality of the meat and be incompatible within the snails' life. The diet is important for the quality of the meat and is characterized by the intake of quality, fresh and selected vegetables represented mainly by: chard, radicchio, kohlrabi, kale, sunflower, Savoy cabbage, and rape. Additional food intake could also be provided by fruits such as sunflower calatis and watermelon, or carrot tubers. The presence of leguminous plants in the seed bed, in addition to food crops, contributes to the maintenance of soil fertility and visibly improves the leaf apparatus of other cultivated species (Brassicaceae). Thus, the aim of this work was to acquire knowledge on the epidemiology and ecology of Aeromonas spp. in snail breeding in Sardinia and to assess the pathogenicity of the different strains isolated from the various environmental matrices. This was done through the molecular characterization of virulence genes, also in order to understand the origin of the contamination for the setting up of preventive actions to improve meat quality.
Methodology
Sampling
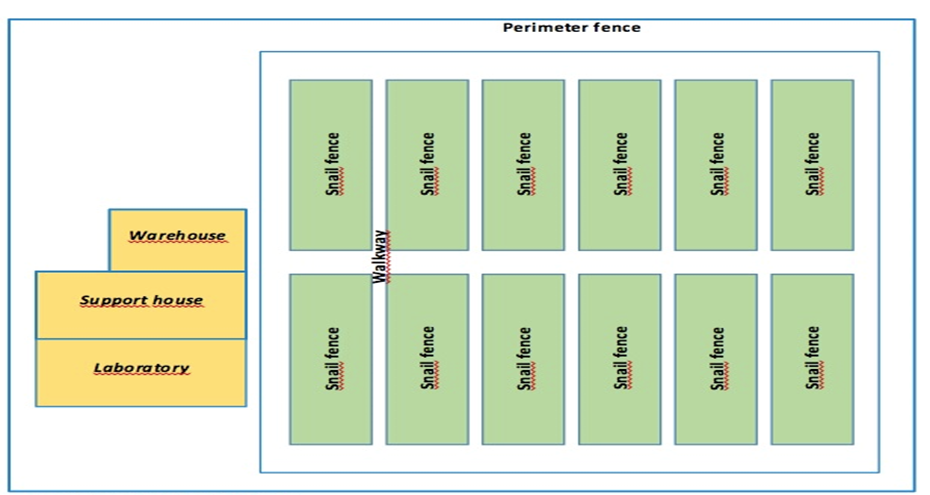
Snail farms (n=8) in the Sardinian territory, with indoor (n.1) and outdoor rearing systems (n.7) (Fig.1,2), samples of snails belonging to Helix aspersa (Müller, 1774) (n= 22) were collected by qualified technicians between June 2020 and November 2021 (Fig.3). At the same time, irrigation water samples (n= 22) from dug wells were collected from the same helicopter farms.
The sampling activity involved the collection of 500 g of adult snails with beading. The samples, uniquely identified and recorded in the sampling report, was deposited in nets, refrigerated and transported to the laboratory.
Sampling equal to 1 L of irrigation water took place immediately prior to entry into the snail farms, after flowing for approximately 1 minute, in sterile bottles. The samples were stored in refrigerated cooler bags at a temperature between 4 and 8° C until arrival at the laboratory.
Microbiological investigations
Microbiological investigations for the detection and enumeration of Aeromonas spp. were conducted on 22 samples of snail meat. From the primary sample of 500g, 50g of meat was taken and 25g was subjected to microbiological investigation according to [15]. Briefly, this method involved the preparation of the initial sample suspension using alkaline peptone water (APA) in a P/V ratio of 1:10 (25g + 225 ml) in sterile food bags subsequently subjected to stomacher for sample homogenization. Scaled dilutions were then set up in sterile saline by transferring 1 ml of each dilution onto three Petri dishes containing Aeromonas agar medium. The inoculum was spread evenly over the surface of the culture medium using a sterile spatula. Once the inoculum was absorbed, the plates were incubated at 37° C ±1 for 24 hours. Then, 5 colonies attributable to Aeromonas spp. were selected and transferred to Klighler Iron Agar (Microbiol Diagnostici, Uta, Cagliari, Italy) and subsequently incubated at 28°C ±1 for 24h. Colonies were then exposed to the oxidase test. On each farm, Aeromonas spp. was tested in the water used for irrigation and animal husbandry purposes. By means of polycarbonate filter membranes with a porosity of 0.45 µm placed in a filtration ramp, 100 ml of water was filtered and the membrane was placed in Aeromonas agar plates incubated at 37°±1 for 24h. The 5 colonies were attributable to Aeromonas spp. were selected and transferred to Klighler Iron Agar and subsequently incubated at 28°±1 for 24h. Colonies were again exposed to the oxidase test.
The colonies that resulted positive for the oxidase test were identified using the API20NE system (Biomerieux). A bacterial suspension of 1 McFarland was inoculated into the API 20 NE (Biomerieux) miniature gallery, humidifying the medium and incubating it at 37°C±1 for 24 h. Subsequently, reading of the tunnel through APIweb and subsequent species identification took place.
Molecular investigations
The biomolecular investigation was performed, by multiplex PCR, on 44 bacterial strains isolated from snail meat and irrigation water samples, for species identification and characterization of the main virulence genes. Genomic DNA extraction was performed from a pure culture using InstaGeneTM Matrix extraction kits (Bio-Rad Laboratories). An isolated colony was dissolved in 200 µl of InstaGeneTM Matrix and placed for 10 minutes at 99 °C. After centrifugation, the supernatant containing the extracted nucleic acid was removed. For all multiplex PCR methods, the reference strain of Aeromonas hydrophila ATCC 7966 was used as a positive control. A negative control (molecular grade water) was included at each run. The samples were subjected to molecular investigation in multiplex PCR for the genes encoding Aeromonas spp. and Aeromonas hydrophila. Primers amplifying a fragment of the 16S rRNA gene, conserved for the genus Aeromonas, were used to confirm the presence of Aeromonas spp. [16] while target sequences of the DNA gyrase subunit B (gyrB), a housekeeping gene, were used to identify the species Aeromonas hydrophila [17, 18]. Strains that tested positive in the initial screening were processed for the characterization of specific virulence factors. These included cytotoxic aerolysin-related enterotoxin (Act), thermolabile cytosolic enterotoxin (Alt), thermostable cytosolic enterotoxin (Ast), haemolysin (HlyA), aerolysin (AerA), elastase (Ela) and lipase (Lip) [19]. The genes were amplified by PCR using oligonucleotide-specific primers (Tab.1) under the conditions of three multiplex PCR reactions (Tab.2) with defined thermal cycles (Tab.3). All primers were synthesized by Sigma Aldrich and the visualization of the amplicons was carried out on 1% agarose gels (Sigma Aldrich, Saint Louis, Missouri,U.S.A.).
Table 1: PCR Primers for Aeromonas genus-specific and virulence-associated
| Target gene | Primer Sequenza 5’ – 3’ | Amplicon size (bp) | Concentration (µM) | Reference |
| A16S F | GGG AGT GCC TTC GGG AAT CAG A | 356 bp | 0.24 | Wang et al, 2003 |
| A16S R | TCA CCG CAA CAT TCT GAT TTG | 0.24 | ||
| A-hyd F | AGT CTG CCG CCA GTG GC | 144 bp | 0.48 | S.Persson et al, 2015 |
| A-hyd R | CRC CCA TCG CCT GTT CG | 0.48 | ||
| astF | ATG CAC GCA CGT ACC GCC AT | 260 bp | 0.12 | Kingombe et al, 2010 |
| astR | ATC CGG TCG TCG CTC TTG GT | 0.12 | ||
| lipF | ATC TTC TCC GAC TGG TTC GG | 382 bp | 0.8 | K.Sen and M.Rodgers, 2004 |
| lipR | CCG TGC CAG GAC TGG GTC TT | 0.8 | ||
| elaF | ACA CGG TCA AGG AGA TCA AC | 513 bp | 0.2 | K.Sen and M.Rodgers, 2004 |
| elaR | CGC TGG TGT TGG CCA GCA GG | 0.2 | ||
GAG AAG GTG ACC ACC AAG AAC A | 232 bp | 0.8 | Kingombe et al, 2010 | |
| actF | ||||
| actR | AAC TGA CAT CGG CCT TGA ACT C | 0.8 | ||
| altF | GCA CGG CGT GAC TTC GGT GA | 576 bp | 0.8 | Kingombe et al, 2010 |
| altR | ACC GCG GTC TTG CAG TTG GG | 0.8 | ||
| aerF | AAC CGA ACT CTC CAT | 301 bp | 1.2 | Pabbs et al, 2009 |
| aerR | CGC CTT GTC CTT GTA | 1.2 | ||
GGC CGG TGG CCC GAA GAT ACG GG | 597 bp | 0.12 | Wong et al, 1998 | |
| hlyAF | ||||
| hlyAR | GGC GGC GCC GGA CGA GAC GGG | 0.12 |
Table 2: Mix Reaction Multiplex PCR 1,2,3.
| Multiplex 1 | Multiplex 2 | Multiplex 3 | |||
| dNTPs | 0,5 µl | dNTPs | 0,5 µl | dNTPs | 0,5 µl |
| A16S-F | 0,5 µl | alt-F | 2 µl | hlyA-F | 0,3 µl |
| A16S-R | 0,5 µl | alt-R | 2 µl | hlyA- R | 0,3 µl |
| A-hyd -F | 0,5 µl | lip-F | 2 µl | ela-F | 0,5 µl |
| A-hyd -R | 0,5 µl | lip-R | 2 µl | ela-R | 0,5 µl |
| ast-F | 0,3 µl | aer-F | 3 µl | ||
| Ast-R | 0,3 µl | aer-R | 3 µl | ||
| act-F | 2 µl | ||||
| act-R | 2 µl | ||||
| H2O PCR | 14,2 µl | 9,2 µl | 6,2 µl | ||
| DNA template | 2 µl | 2 µl | 2 µl | ||
| Taq Polymerase | 0,2 µl | 0,2 µl | 0,2 µl | ||
| MgCl2 | 2 µl | 2 µl | 2 µl | ||
| Buffer | 2,5 µl | 2,5 µl | 2,5 µl | ||
| Volume totale | 25 µl | 25 µl | 25 µl | ||
Table 3: Multiplex 1,2,3 PCR: thermic profiles
| Step | Temperature | Time (Min) | Cicle |
| Initial denaturization | 95°C | 05:00 | 1 |
| Denaturisation | 95°C | 00:30 | |
| Coupling | 60°C | 00:30 | 30 |
| Extension | 72°C | 01:00 | |
| Final Extension | 72°C | 07:00 | 1 |
Table 4: Detection enterotoxin gene in isolates of Aeromonas hidrophila and spp in the meat samples
| Alt | Lip | Ast | Hly | Ela | Aer | Act | ||
| 1C | A. h | + | + | - | + | + | - | - |
| 2C | A. spp | - | - | - | - | - | - | + |
| 3C | A. spp | - | + | - | + | - | - | + |
| 4C | A. h | + | + | + | + | + | - | - |
| 5C | A. h | + | + | - | - | + | - | + |
| 6C | A. h | + | + | - | + | + | - | - |
| 7C | A. spp | - | + | - | - | + | - | - |
| 8C | A. h | + | + | - | + | + | - | - |
| 9C | A.spp | - | + | - | + | - | - | + |
| 10C2 | A. spp | - | - | - | - | - | - | + |
| 10C1 | A. h | + | + | + | - | + | - | - |
| 13C | A. spp | - | + | - | - | + | - | - |
| 11C | A. h | + | + | - | + | + | - | - |
| 12C | A. h | + | + | + | + | + | - | - |
| 14C | A. spp | - | + | - | - | + | - | - |
| 17C | A. spp | - | + | - | + | - | - | + |
Discussion and Results
Overall, 61% of the meat and water samples tested for detection and enumeration of Aeromonas spp. (n=19, n= 8 meat, n=11 water, A.sobria and caviae) and Aeromonas hydrophila (n=8) only in the meat were positive with an average concentration of 4.8 and 4.7 logs respectively, while the results of the other samples were below the detection limit of the method (<10>Aeromonas hydrophila isolates was constant for the Alt, Lip, Ela genes (n. 8/8 samples), while the Ast (n.3/8 samples), Hly (n.6/8 samples) and Act genes were present in one sample. Aer gene was not detected (Tab. 4).
The presence of cytotoxic enterotoxins such as alt and ast may present an important aspect in the virulence expression of the strains tested, as they are similarly to cholera toxin by raising the levels of adenylated cAMP and prostaglandins in intestinal epithelial cells [17,18]. In samples with Aeromonas spp. isolates, the virulence genes alt, ast, aer were never detected, the lip gene in six samples, act gene five samples, the Ela and Hly genes in three samples were present of the isolates. Moreover, 46% of the water samples tested were only positive for Aeromonas spp. (n=10) with 1 sample showing a concentration of 2.2 log, 4 samples showing a concentration of 2.7 log, 3 samples showing a concentration of 3.5 log and only 1 sample showing a concentration of 4.2 log. The rest of the samples were below the detection limit of the method (<1>Aeromonas sobria and caviae.
All the microorganisms isolated showed the presence of a single but relevant virulence gene (act). Cytotoxic enterotoxin (act) is, in fact, the main virulence factor of A. hydrophila and Aeromonas spp. and is responsible for the haemolytic, cytotoxic and enterotoxic activities. Haemolytic activity occurs with a haemolysin that through the formation of pores from 1.14 to 2.8 nm in the erythrocyte membrane allows the entry of water from the external medium resulting in cell lysis [5,6]. In the samples examined, most of the Aeromonas strains showed virulence genes and thus a potential expression of extracellular enzymes, such as protease, lipase, collagenase, nuclease, amylase, chitinase and elastase. Analysis of the presence of virulence genes in the meat of the snails and in the irrigation water of the snail plants examined reveals a mismatch in the presence of virulence genes between the different strains isolated. This suggests that the origin of Aeromonas contamination in snails does not originate from these waters but rather from other reservoirs or sources of contamination such as supplementary feeding sources represented by vegetable mowings of various origins and sources. Indeed, studies have found that Aeromonas spp. in water used for agricultural irrigation may pose a risk as a source of contamination of bacteria in the food chain and thus in snail plants [20,21]. This can contaminate the surface of vegetables and together with the lack of proper hygiene at various stages of the chain can contribute to the spread of Aeromonas spp. [13]. Several studies have reported the presence of Aeromonas spp. in different vegetable species (cabbage, carrot, cucumber, aubergine, lettuce, onion, tomato, potato and spinach) with Aeromonas caviae being the most prevalent species followed by A. hydrophila [9,10,11,12]. In addition to the pathogenetic mechanisms of Aeromonas spp. in humans with virulence factors contributing to biofilm formation, cell adherence, invasion and cytotoxicity, the literature confirms that Aeronomas can severely damage the production of snail farms [3,5].
Conclusion
This study revealed that Aeromonas spp. was frequently detected in Snail farms in Sardinia, in particular within isolated snail meat and in water used for zootechnical purposes in different seasons. The study underlines a critical control point for minimising the risk of Aeromonas ssp. contamination by adopting appropriate management and microbiological monitoring of supplementary food sources from outside environments, which are often irrigated with insufficiently controlled water.
Declarations
Contributions: The authors contributed equally.
Conflict of interest: The authors declare no potential conflict of interest.
Funding: None.
Availability of data and material: Data and materials are available by the authors.
References
- Rhee, J. Y., Jung, D. S., & Peck, K. R. (2016). Clinical and therapeutic implications of Aeromonas bacteremia: 14 years nation-wide experiences in Korea. Infection & Chemotherapy, 48(4), 274-284.
View at Publisher | View at Google Scholar - Hoel, S., Vadstein, O., & Jakobsen, A. N. (2017). Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Frontiers in Microbiology, 8, 931.
View at Publisher | View at Google Scholar - Kodjo, A., Haond, F., & Richard, Y. (1997). Molecular and phenotypic features of aeromonads isolated from snails (Helix aspersa) affected with a new summer disease. Journal of Veterinary Medicine, Series B, 44(1‐10), 245-252.
View at Publisher | View at Google Scholar - Abuhammour, W., Hasan, R. A., & Rogers, D. (2006). Necrotizing fasciitis caused by Aeromonas hydrophilia in an immunocompetent child. Pediatric emergency care, 22(1), 48-51.
View at Publisher | View at Google Scholar - Tomás, J. M. (2012). The main Aeromonas pathogenic factors. International Scholarly Research Notices, 2012.
View at Publisher | View at Google Scholar - Sha, J., Kozlova, E. V., & Chopra, A. K. (2002). Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infection and Immunity, 70(4), 1924-1935.
View at Publisher | View at Google Scholar - Chakraborty, T. M. S. R. E. K., Montenegro, M. A., Sanyal, S. C., Helmuth, R., Bulling, E., & Timmis, K. N. (1984). Cloning of enterotoxin gene from Aeromonas hydrophila provides conclusive evidence of production of a cytotonic enterotoxin. Infection and Immunity, 46(2), 435-441.
View at Publisher | View at Google Scholar - Bhowmick, U. D., & Bhattacharjee, S. (2018). Bacteriological, Clinical and Virulence Aspects of-associated Diseases in Humans. Polish Journal of Microbiology, 67(2), 137-150.
View at Publisher | View at Google Scholar - Abbey, S. D., & Etang, B. B. (1988). Incidence and biotyping of Aeromonas species from the environment. Microbios, 56(228-229), 149-155.
View at Publisher | View at Google Scholar - Latif-Eugenín, F., Beaz-Hidalgo, R., Silvera-Simón, C., Fernandez-Cassi, X., & Figueras, M. J. (2017). Chlorinated and ultraviolet radiation-treated reclaimed irrigation water is the source of Aeromonas found in vegetables used for human consumption. Environmental research, 154, 190-195.
View at Publisher | View at Google Scholar - Nishikawa, Y., & Kishi, T. (1988). Isolation and characterization of motile Aeromonas from human, food and environmental specimens. Epidemiology & Infection, 101(2), 213-223.
View at Publisher | View at Google Scholar - Nagar, V., Shashidhar, R., & Bandekar, J. R. (2011). Prevalence, characterization, and antimicrobial resistance of Aeromonas strains from various retail food products in Mumbai, India. Journal of food science, 76(7), M486-M492.
View at Publisher | View at Google Scholar - Adebayo, E. A., Majolagbe, O. N., Ola, I. O., & Ogundiran, M. A. (2012). Antibiotic resistance pattern of isolated bacterial from salads. Journal of Research in Biology, 2(2), 136-142.
View at Publisher | View at Google Scholar - Coroneo, V., Marras, L., Giaccone, V., Conficoni, D., Brignardello, S. A. S., Bissacco, E., ... & Cogoni, M. P. (2022). Evaluation of the microbiological and chemical aspects of autochthonous wild snails in Sardinia. Italian Journal of Food Safety, 11(2).
View at Publisher | View at Google Scholar - Rapporti ISTISAN 07/5 (2007). https://www.salute.gov.it/imgs/C_17_pubblicazioni_2278_allegato.pdf
View at Publisher | View at Google Scholar - Rather, M. A., Willayat, M. M., Wani, S. A., Munshi, Z. H., & Hussain, S. A. (2014). A multiplex PCR for detection of enterotoxin genes in Aeromonas species isolated from foods of animal origin and human diarrhoeal samples. Journal of applied microbiology, 117(6), 1721-1729.
View at Publisher | View at Google Scholar - Persson, S., Al-Shuweli, S., Yapici, S., Jensen, J. N., & Olsen, K. E. (2015). Identification of clinical aeromonas species by rpoB and gyrB sequencing and development of a multiplex PCR method for detection of Aeromonas hydrophila, A. caviae, A. veronii, and A. media. Journal of clinical microbiology, 53(2), 653-656.
View at Publisher | View at Google Scholar - Yang, Y., Yu, H., Li, H., Wang, A., & Tan, S. (2017). Multiplex taqman real-time PCR for detecting Aeromonas hydrophila, A. veronii and A. schubertii.
View at Publisher | View at Google Scholar - Zhou Y, Yu L, Zheng N, Zhang P, Kan B, Yan D and Su J. (2019) Taxonomy, virulence genes and antimicrobial resistance of Aeromonas isolated from extra-intestinal and intestinal infections
View at Publisher | View at Google Scholar - Pianetti, A., Sabatini, L., Bruscolini, F., Chiaverini, F., & Cecchetti, G. (2004). Faecal contamination indicators, Salmonella, Vibrio and Aeromonas in water used for the irrigation of agricultural products. Epidemiology & Infection, 132(2), 231-238.
View at Publisher | View at Google Scholar - Al-Jassim, N., Ansari, M. I., Harb, M., & Hong, P. Y. (2015). Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation?. Water research, 73, 277-290.
View at Publisher | View at Google Scholar