Research Article | DOI: https://doi.org/10.58489/2836-2284/006
A new test (Sacco Manni test 3) of congenital dysplasia of the HIP: A Cross Sectional and Analytic Study
Istitution: Asl Bari, Italy
Department: Orthopaedic and Trauma Surgery
Address: Viale Regina Margherita, 70022, Altamura (Bari), Italy.
*Corresponding Author: Saccomanni Bernardino
Citation: Saccomanni Bernardino, (2022). A new test (Sacco Manni test 3) of congenital dysplasia of the HIP: A Cross Sectional and Analytic Study. Journal of Skeleton System (JSS). 1(1). DOI: 10.58489/2836-2284/006.
Copyright: © 2022 Saccomanni Bernardino, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 28 October 2022 | Accepted: 15 November 2022 | Published: 18 December 2022
Keywords: new test; congenital dysplasia of hip
Abstract
Purpose Or Objective: Without a prompt diagnosis, congenital dysplasia of the hip in newborns can lead severe sequelae. Current screening strategies emphatize the use of ORTOLANI AND BARLOW tests, yet they exhibit low sensibility. The purpose of this study is to evaluate for a new test as a screening tool for congenital dysplasia of the hip.
Methods: to evaluate the new test, a cross sectional and analytic study was performed with a non-probabilistic sampling method. Patients with either a positive ORTOLANI AND BARLOW TESTS were evaluated with the new test (Sacco Manni test 3) and hip ultrasounds. Controls were infants
With negative ORTOLANI AND BARLOW and SACCOMANNI TESTs and also had an ultrasound performed.
Results: CONGENITAL dysplasia of the hip was confirmed in 83 to 130 cases (64%), and 2 of 130 controls (2%). The new test had a sensitivity of 76% AND A specificity of 94% as compared to the ORTOLANI AND BARLOW TESTS (sensitivity 31 to 32 %, specificity 93 to 100% (p inferior to 0,05).
Conclusion: this new test could serve as another clinical tool for the initial screening of congenital dysplasia of the hip in in fans or newborns.
Its promising results against traditional screening procedures might potentially impact diagnosis and prognosis for patients with congenital dysplasia of hip.
Introduction
CONGENITAL dysplasia of the hip refers to a wide spectrum of abnormal hip development in newborns, namely secondary to abnormal coaptation between the acetabulum and femoral head (1).
Without a prompt diagnosis, the integrity of the joint and its biomechanics is often compromised, which can lead to great sequelae or a residual dysplasia that can contribute to an early onset osteoarthrosis (2).
The most widely used tests in physical exanimation to screen for congenital dysplasia of the hip are ORTOLANI AND BARLOW.
ALTHOUGH their specificity is high (ranging between 93 and 96%), their sensitivity (estimated to be around 26 to 28%) is quite low (2).
The latter possesses a significant disadvantage, leading a high rate of diagnostic delays.
New, noninvasive, and low-risk diagnostic methods are necessary to amplify for reach of current screening procedures for congenital dysplasia of the hip.
The objective of this study is to evaluate the performance of ORTOLANI AND BARLOW in a population of infants or newborns and compare their performance with the sensibility and specificity of a novel test proposed in this study.
Methods
To evaluate the new test, a cross sectional and analytical study was performed. The sample size (n) was estimated to be 42 patients per group, taking into consideration a national performance of congenital dysplasia of hip of 0,3% (3) (p) with a level of confidence of 95% (z). The sample method was nonprobabilistic.
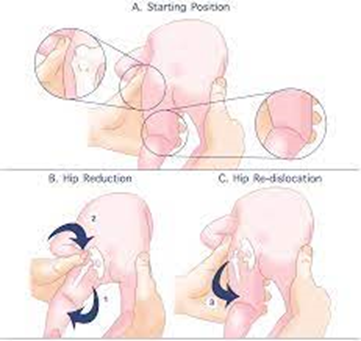
The new test is shown in figure 1.
- Starting position. With new born in a prone position, the hips and knees are flexed
90° degrees in adduction. In this position, the exploring physicians’ places both thumbs over the major thochanter of the femur in the infant’s hip and the rest of fingers over the knees so a complete control of the whole femur is achieved with the physician’s hand.
B. Hip reduction
C. Hip re-dislocation
Potential study candidates with a positive Ortolani or Barlow test
During birth hospitalization were identified and invited to participate.
After written informed consent was obtained, the new test was performed by the principal investigator and the results were documented
In word file, ensuring at all times data was codified to protect the minor’s identity. Thereafter, A referral was placed to the hospital’s orthopedics and traumatology department, where patients were evaluated with a hip ultrasound to confirm a diagnosis of congenital dysplasia of hip.
Controls were infants who, during their birth hospitalization or a clinic visit, were evaluated with BARLOW, ORTOLANI AND THE NEW TEST
With negative results and were also evaluated with hip ultrasound.
STATISTICAL ANALYSIS AND VARIABLES
THE statistical program used for data analysis was SPSS V. 25.
Sensitivity, specificity, positive predictive value (PPV), and negative
Predictive value (NPV) WERE OBTAINDED for each test performed-ORTOLANI, BARLOW AND THE NEW TEST.
Interobserver reliability of the tests was also measured with alpha krippend off coefficient which is a statistical measure of the extent of agreement among coders, where k “1” presents perfect reliability and k “1” absence of reliability.
Results
Over of period of 2 years (from 2001 to 2003), 179 patients with a positive ORTOLANI OR BARLOW TEST were identified at the hospital and invited to enter the study.
Forty-nine were lost to follow-up, so the final number of enrolled patients with a positive test 130.
There were 130 controls identified.
Eighty three of 130 cases (64%) and 2 of 130 controls (2%) were confirmed to have congenital dysplasia of hip by means of hip ultrasound.
THE sensitivity, specificity, PPV, NPV AND krippend off alpha costant for the ORTOLANI, BARLOW AND THE NEW TEST ARE GIVEN IN TABLE 1.
Table 1.
Sensitivity, Specificity, Ppv and Npv for Ortolani, Barlow and The New Test for Diagnoses and Congenital Dysplasia of Hip as Confirmed by Ultrasound
| SENSITIVITY | SPECIFICITY | PPV | NPV | K |
BARLOW | 31% | 100% | 100% | 29% | 0.16 |
ORTOLANI | 32% | 93% | 90% | 39% | 0.18 |
NEW TEST | 76% | 95% | 84% | 92% | 0.73 |
The new test was more sensitive than ORTOLANI AND BARLOW, and had Higher interobserver agreement (k=0.8) (p inferior to 0.05).
Discussion
Secondary prevention strategies aimed at early detection are corner store of effective treatment.
This is important given that unrecognized cases of dysplastic hip are estimated to be the most common cause of early onset osteoarthritis
Of the hip in young females, and accountable for up to 5 to 10% of hip replacements performed in the UNITED STATED ALONE (4).
MOREOVER, the impact of long-standing dysplasia on the individual’s physic social well-being, quality of life, and workforce productivity
Are far from negligible (5) and further stress the importance of preventive measures.
SCREENING protocols vary by country, but most countries including Italy Rely on the general recommendations established by the American academy of pediatrics (4), which consist of periodic ORTOLANI AND BARLOW TESTS at birth and at schedulated well-child care visits.
The new test proposed in this study showed a sensitivity of 76%, Specificity of 95% (p inferior to 0.001), and a favorable inter-observer Reability (0.73), not far behind the sensitivity (88.8%) and specificity (98.7%) of hip ultrasound (6), the current gold standard for diagnosis.
The new test could serve as another clinical tool for the initial screening of congenital dysplasia of hip in newborns.
Its use in routine newborn examinations could rapresent a turning point in diagnosis and prognosis for patients with congenital dysplasia of hip.
Multicenter studies are necessary to validate the external validity of the test.
References
- YANG S. ET AL (2019): Development dysplasia of the hip, PEDIATRICS JOURNAL; 143(1): 100-4.
View at Publisher | View at Google Scholar - JIMENEZ C. AT AL (994): Validity and diagnostic bias in the clinical screening for congenital dysplasia of the hip; Acta orthop. Belg.1; 60(3): 315-21.
View at Publisher | View at Google Scholar - CLARO-HERNANDEZ ET AL (2017): Epidemiologia de la dysplasia del desarrollo de la cadera. REV. ESP MED QUIR; 22(17): 22-7
View at Publisher | View at Google Scholar - SHAW BA (2016) et al: Evaluation and referral for development dysplasia of the hip in infants, PEDIATRICS, 138(6): 99-103;
View at Publisher | View at Google Scholar - OKEN FO (2018) et al: Factors affecting the return to work of total hip arthroplasty due to of development hip dysplasia in young patients; j. orthop.; 15(2): 450-4
View at Publisher | View at Google Scholar - WOOLACOTT NF ET AL (2005): Ultrasonography in screening for development Dysplasia of the hip in newborns: systematic review. BRITISH MED. J.; 330(7505):1413-5.
View at Publisher | View at Google Scholar